Beta thalassemia major (BTM) is an autosomal recessive disorder caused by a severely decreased synthesis of normal beta globin chains. The widely used therapy for this disorder is regular red blood cell transfusion, which alleviates anemia and suppresses ineffective erythropoiesis.1 However, lifetime transfusion therapy causes iron overload and hemosiderosis in different organs (i.e., heart, liver, kidneys) so that cardiac hemosiderosis is the most common cause of death in these patients.2 The mortality rate may be related to the side effect of iron chelation therapy used for treatment of organ-specific hemosiderosis.3
Despite receiving regular and intense iron chelation therapy, the iron overloading complications in some patients with BTM is more serious and more frequent than in others. We hypothesized that the coexistence of some genetic variations modifying iron metabolism in patients with BTM may contribute to the variability of phenotypic expression of iron overloading complications in these patients.4 Genetic variations in the genes responsible for iron homeostasis can aggravate the complications of iron overload and accelerate the development of organ-specific hemosiderosis in patients with BTM.4 HFE C282Y and H63D mutations are the common causes of iron overload in different populations.5 The HFE C282Y mutation reduces the cell surface expression of protein thereby preventing the HFE molecule from interacting with β2-microglobulin.6 The HFE H63D mutation is due to a C-to-G change at nucleotide 187 converts histidine (H) at position 63 to aspartic acid (D) in the HFE protein. The mutant D allele causes a defective interaction between HFE molecule, β2-microglobulin and transferrin receptor on the cell surface, which can lead to increased iron absorption and hemosiderosis.6–9
The frequency of two HFE gene mutations (C282Y and H63D) was reported to be high in Caucasian and Indian population.9 No comprehensive study exists looking at the prevalence of these two HFE gene mutations in the Iranian population. However, one study of Iranian patients found that the HFE C282Y gene mutation was not present in any of 50 normal control subjects whereas the HFE H63D gene mutation was detected in 26% of control subjects.10 The HFE H63D and C282Y mutations were found to be associated with elevated serum iron and ferritin levels in patients with BTM. This may confer increased susceptibility to the development of organ-specific hemosiderosis in these patients.4,6,7 However, studies investigating the role of HFE C282Y and H63D gene mutations in the development of cardiac and hepatic hemosiderosis in patients with BTM are limited, especially in the Iranian population.11–14 Moreover, accurate assessment of iron overload and organ-specific hemosiderosis in patients with BTM may play important roles in managing these patients better. The principal method to assess iron loading includes measurement of ferritin levels and the liver iron concentration (LIC) assay.15,16 Moreover, T2* magnetic resonance imaging (MRI) as a non-invasive method is currently the gold standard approach for evaluating organ-specific hemosiderosis.16,17 However, T2*MRI is expensive, not widely available, and its interpretation needs an expert radiologist. Also, MRI scan is not an easy procedure in children. Many studies have investigated the correlation between plasma ferritin levels and T2*MRI values of heart and liver to determine whether ferritin levels could be used as a suitable index to assess iron overload status in such patients. However, conflicting results have been reported.18–22 Our study aimed to investigate whether the HFE H63D and C282Y gene mutations could contribute to the development of cardiac and hepatic hemosiderosis in patients with BTM. We also investigated the association of these mutations with some biochemical iron markers and the correlation of hepatic and cardiac T2* MRI values and LIC with each other and plasma ferritin levels.
Methods
Between February and September 2016, we conducted a cross-sectional study of 60 patients with BTM who were admitted to the Mousavi Hospital of the Zanjan province, Iran. Twenty-eight women and 32 men with a mean age of 17.5±9.1 years were enrolled. Patients with BTM (diagnosed by molecular methods) who were on regular red blood cell transfusion (at least two per month) and received chelation therapy were included in the study. Patients suffering from hepatitis B or hepatitis C infection or any other disease such as malignancy, renal disease, inflammation, and autoimmune disorders were excluded. Patients with thalassemia minor and intermedia or patients who refused to be part of the study were excluded. Written informed consent was obtained from all participants, and the study was approved by the ethical committee of Zanjan University of Medical Science (Ethical committee code: ZUMS.REC.1393.166).
Table 1: Cardiac and hepatic T2*MRI findings. Data presented as n (%) unless otherwise indicated.
|
T2* Heart, ms |
Mean±SD |
23.8 ± 12.1 |
|
Cardiac hemosiderosis |
No |
37 (61.6) |
|
Yes |
23 (38.3) |
|
Severe |
6 (10.0) |
|
Moderate |
9 (15.0) |
|
Mild |
8 (13.3) |
|
T2* Liver, ms |
Mean±SD |
8.3 ± 8.7 |
|
Hepatic hemosiderosis |
No |
35 (58.3) |
|
Yes |
25 (41.6) |
|
Severe |
8 (13.3) |
|
Moderate |
9 (15.0) |
|
Mild |
8 (13.3) |
MRI: magnetic resonance imaging; SD: standard deviation.
Fasting blood samples were collected from all patients in ethylenediaminetetraacetic acid (EDTA) containing tubes. Plasma iron and transferrin levels were measured by routine colorimetric methods using commercially available kits (Pars. Azmoon Ltd., Tehran, Iran). Transferrin saturation index was calculated by the ratio of plasma iron and transferrin levels multiplied by 100. Ferritin levels were determined by the enzyme immune assay (ELISA kit, Pishtaz Teb Ltd, Tehran, Iran) according to the manufacturer’s protocol. For genotyping of HFE H63D and C282Y mutations, DNA was extracted from blood leukocytes using a commercially available kit (Geno Plus Genomic DNA Mini, Viogene, Poland) according to the manufacturer’s instructions. Detection of HFE H63D and C282Y mutations was conducted using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method using BclI and RsaI restriction enzymes as described previously.23 The amplicon size of HFE H63D mutation was 207 bp, and its digestion with BclI enzyme results in 138 bp and 69 bp fragments in the presence of H allele and an undigested 207 bp fragment in the presence of D allele. The amplicon size of HFE C282Y mutation was 390 bp, and its digestion with RsaI enzyme results in 250 bp and 140 bp fragments in the presence of 282C allele and 250 bp, 111 bp and 29 bp in the presence of 282Y allele. All patients underwent MRI using a 1.5 Tesla scanner (Achieva 1.5T A-series, Philips Medical Systems) at Pardis Noor Clinic, Tehran, Iran. The protocol used for T2*MRI measurements in all patients was based on the Royal Brompton procedures utilizing a single breath, multi-echo, fast gradient-echo sequence. All patients were categorized into four groups based on their T2*MRI milliseconds (ms) results according to following cutoff points; cardiac hemosiderosis: normal > 20 ms, mild: 14–20 ms, moderate: 10–14 ms, severe < 10 ms; hepatic hemosiderosis: normal > 6.3 ms, mild: 2.8–6.3 ms, moderate: 1.4–2.7 ms, severe < 1.4 ms; LIC: normal > 2 ms, mild: 2–5 ms, moderate: 5–10 ms, severe > 10 ms. Statistical analysis was done using SPSS Statistics (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.). Categorical variables were presented as numbers and percentages and were analyzed using the chi-square test. Quantitative variables were presented as means and standard deviation (SD) and analyzed using the Mann-Whitney U test. Correlation analysis was done using the Spearman’s test. A p-value < 0.050 was considered statistically significant.
Table 2: Association between hepatic and cardiac hemosiderosis and some biochemical iron markers in patients with beta thalassemia major. Data presented as mean±SD.
|
Hepatic hemosiderosis |
17.3 ± 9.6 |
1633.8 ± 414.5 |
219.4 ± 51.5 |
59.6 ± 17.6 |
|
Non-hepatic hemosiderosis |
17.6 ± 9.8 |
1162.1 ± 723.4 |
158.1 ± 56.7 |
45.8 ± 12.5 |
|
p-value |
0.916 |
0.021 |
0.001 |
0.010 |
|
Cardiac hemosiderosis |
22.7 ± 7.7 |
1710.7 ± 463.2 |
231.6 ± 48.5 |
61.1 ± 16.5 |
|
Non-cardiac hemosiderosis |
14.2 ± 9.4 |
1130.0 ± 663.7 |
153.8 ± 56.1 |
47.6 ± 13.1 |
SD: standard deviation; TSI: transferrin saturation index.
Results
The investigated population was 60 BTM patients including 28 female and 32 men who were on regular red blood cell transfusion at intervals of 2–4 weeks. The mean ages of the BTM patients were 17.5±9.1 years. The rate of splenectomy in the study population was 25.0% (15 out of 60 BTM patients). Of the 60 patients, 10 (16.6%) had developed alloantibody with anti-K the most common. All patients were transfused with leukoreduced packed red blood cells. All patients received regular iron chelation therapy from early childhood. Thirty-five patients were under regular iron chelation therapy with desferrioxamine (30–50 mg/kg/day), which was administered subcutaneously. The 25 patients that had an intolerance to desferrioxamine were chelated with oral iron chelator deferasirox (30–40 mg/kg/day). Abnormal hepatic iron load (T2*MRI < 6.3 ms) and abnormal cardiac iron load (T2*MRI < 20 ms) was detected in 25 (41.6%) and 23 (38.3%) patients with BTM, respectively. Detailed information regarding the cardiac and hepatic T2*MRI values in the BTM patients are presented in Table 1. The comparison of BTM patients with and without hemosiderosis revealed that plasma ferritin levels, iron levels, and transferrin saturation index were significantly higher in BTM patients with hepatic hemosiderosis than those without hepatic hemosiderosis (p = 0.021; p = 0.001; p = 0.010, respectively). Similarly, these values were significantly higher in BTM patients with cardiac hemosiderosis than those without cardiac hemosiderosis (p = 0.008; p = 0.001; p = 0.012, respectively) [Table 2].
The correlation between plasma ferritin levels and hepatic T2*MRI values indicated a statistically significant inverse correlation (r = -0.631, p = 0.001) [Figure 1a]. Plasma ferritin levels showed a strong direct correlation with LIC (r = 0.701, p = 0.007). A strong inverse correlation was seen between the hepatic T2*MRI values and LIC (r = -0.812, p < 0.001) [Table 3]. However, the inverse correlation between plasma ferritin levels and cardiac T2*MRI values was poor (r = -0.297, p = 0.044) [Figure 1b]. Also, the inverse correlation between cardiac T2*MRI values and LIC was poor and insignificant (r = -0.285, p = 0.270). Moreover, the correlation between cardiac T2*MRI and hepatic T2*MRI values was also weak and insignificant (r = 0.287, p = 0.058) [Table 3]. The correlation of age with cardiac T2*MRI values in patients with BTM indicated a significant and moderate inverse correlation (r = -0.519, p = 0.017). However, the correlation of age with hepatic T2*MRI values (r = -0.167, p = 0.312) and LIC (r = 201, p = 0.075) was poor and statistically insignificant [Table 4].
Table 3: Correlation between ferritin and T2*MRI values of heart and liver in patients with BTM.
|
0.007 |
0.544 to 0.810 |
0.701 |
LIC, mg/g/dry weight |
Ferritin |
|
0.270 |
-0.034 to -0.502 |
-0.285 |
LIC, mg/g/dry weight |
Cardiac T2*MRI, ms |
|
< 0.001 |
-0.704 to -0.883 |
-0.812 |
LIC, mg/g/dry weight |
Hepatic T2*MRI, ms |
BTM: beta thalassemia major; LIC: liver iron concentration; CI: confidence interval; MRI: magnetic resonance imaging; ms: milliseconds.
Table 4: Correlation of age with ferritin and T2*MRI values of the heart and liver in patients with BTM.
|
0.017 |
-0.423– -0.629 |
-0.519 |
Cardiac T2*MRI, ms |
Age |
|
0.312 |
-0.112– -0.231 |
-0.167 |
Hepatic T2*MRI, ms |
Age |
|
0.075 |
1.08–3.14 |
0.201 |
LIC, mg/g/dry weight |
Age |
|
BTM: beta thalassemia major; LIC: liver iron concentration; CI: confidence interval; MRI: magnetic resonance imaging. |
We investigated the genotype distribution of HFE C282Y and H63D mutations in our cohort. The HFE C282Y mutation was not detected in any patients in our study. However, the HFE H63D mutation was detected in 12 (20.0%) patients. The genotype distribution of HFE H63D mutation (HH = 48, HD = 10, DD = 2) was in accordance with the Hardy-Weinberg equilibrium (p = 0.138), indicating the absence of selection bias in our study. As shown in Table 5, the carrier frequency of HFE H63D mutation did not differ significantly between patients with and without hepatic hemosiderosis [odd ratio (OR) = 2.33; 95% confidence interval (CI): 0.64–8.45; p = 0.190] as well as between patients with and without cardiac hemosiderosis [OR = 1.82; 95% CI: 0.50–6.52; p = 0.350] [Table 5]. However, comparing patients with and without HFE H63D mutation indicated significantly higher plasma ferritin levels (1 903±993 vs. 992±683, p < 0.001) and iron levels (214.56±39.46 vs. 167.83±58.61, p = 0.008) in carriers of HFE 63D allele relative to carriers of HFE 63H allele. No significant differences were seen regarding the mean transfused blood (mL/kg/year) (215.9±54.6 vs. 208.4±60.2, p = 0.190), mean age (17.8±9.7 vs. 17.4±9.6 years) and splenectomy rate (33.3% vs. 22.9%, p = 0.270) between the two patient subgroups.
Table 5: The association between hepatic and cardiac hemosiderosis and HFE H63D mutation in patients with BTM. Data presented as n (%) unless otherwise indicated.
|
Hepatic hemosiderosis patients, n = 25 |
18 (72.0) |
6+1 (28.0) |
42 (84.0) |
8 (16.0) |
|
Non- hepatic hemosiderosis patients, n = 35 |
30 (85.7) |
4+1 (14.2) |
64 (91.4) |
6 (08.5) |
|
p-value |
0.190 |
0.210 |
|
OR (95% CI) |
2.33 (0.64–8.45) |
2.03 (0.65–6.27) |
|
Cardiac hemosiderosis patients, n = 23 |
17 (73.9) |
5+1 (26.0) |
39 (84.7) |
07 (15.2) |
|
Non-cardiac hemosiderosis patients, n = 37 |
31 (83.7) |
5+1 (16.2) |
67 (90.5) |
07 (09.4) |
|
p-value |
0.350 |
0.340 |
BTM: beta thalassemia major; HH: wild-type genotype; HD: homozygous genotype; DD: homozygous genotype; H: wild allele; D: mutant allele; OR: odds ratio; CI: confidence interval.
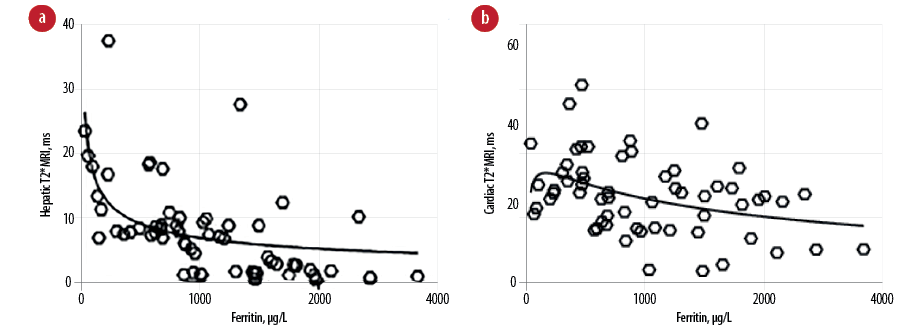
Figure 1: Correlation between plasma ferritin levels and (a) hepatic and (b) cardiac T2* magnetic resonance imaging (MRI), ms values.
Discussion
Cardiac and hepatic hemosiderosis are the main causes of morbidity and mortality in patients with BTM. The results of our study indicated cardiac and hepatic hemosiderosis in 38.3% and 41.6% of the patients, respectively. Different rates of cardiac and hepatic hemosiderosis were reported in previous studies.20,22,24 Mean age differences of the studied populations, variations in sample size, and of chelation regimes may considerably affect the incidence of cardiac and hepatic hemosiderosis in different studies.19,24 Interestingly, it is known that deferiprone results in preferential cardiac chelation whereas deferoxamine may be more effective for hepatic chelation.19 Also, our results are in accordance with some previously published studies that demonstrated a statistically significant inverse correlation between plasma ferritin levels and hepatic T2*MRI values (p = 0.001, r = -0.631) and indicated that plasma ferritin levels could accurately and thoroughly estimate hepatic iron load.20–22 This result may be of great importance in unequipped centers where T2*MRI is unavailable. However, other studies have reported different correlation strengths ranging from no correlation to moderate correlation.18,25,26
Our study indicated a weak correlation between plasma ferritin levels and cardiac T2*MRI values that was in accordance with previous studies.21,26,27 However, in contrast to our result, Yang et al,19 in a recently published study reported a statistically significant strong correlation between serum ferritin levels and cardiac T2*MRI values. This may be explained by the fact that the vast majority of patients (97.5%) included in their study received poor chelation therapy. Similar to previous studies,18,26,28 our study indicated a poor and statistically insignificant correlation between cardiac and hepatic T2*MRI values (p = 0.058, r = 0.287). The poor correlation of cardiac T2*MRI values with plasma ferritin levels as well as with hepatic T2*MRI values strongly suggest that plasma ferritin levels and hepatic T2*MRI values cannot thoroughly predict cardiac iron content. This finding emphasizes the importance of using cardiac T2*MRI as a non-invasive and precise procedure for estimating of cardiac iron overload instead of relying solely on ferritin levels or hepatic T2*MRI values. The possible mechanisms for poor or insignificant correlation between hepatic and cardiac T2*MRI values may be related to the organ-specific mechanisms of iron uptake and release.29 Also chelation therapy is usually more effective in eliminating iron from the liver than heart.19 Another important aspect of our study was that a statistically significant moderate correlation was seen between age and cardiac T2*MRI values while the correlation of age with hepatic T2*MRI values was weak and insignificant. Similar results have been reported previously.18,19,28 Since the liver is the first organ to store iron in the body, most patients develop hepatic iron overload earlier in life. However, cardiac iron overload develops slowly in a time-dependent manner so that it progresses as the patient ages. Therefore, the age of patients has a more pronounced impact on the development of cardiac hemosiderosis relative to the development of hepatic hemosiderosis. The study by Yang et al,19 proposed that cardiac hemosiderosis should be assessed using T2*MRI in patients with BTM at six years of age. Iron overload cardiomyopathy (IOC) is the serious manifestation of iron deposition in the heart. The spectrum of symptoms of IOC ranges from asymptomatic in the early stage of disease to terminal heart failure in severely overloaded patients. Early diagnosis and adequate medical therapy can reverse IOC and has a crucial role in the prevention and treatment of this disorder.30 Our results showed that MRI is more sensitive and more precise than analysis of routine biochemical markers and we would like to reemphasize the advantage of using this technique for the early detection of cardiac iron overload in patients with BTM.
HFE H63D and C282Y gene mutations are the common causes of genetic hemosiderosis. Interestingly, some previous studies have reported an increased frequency of HFE gene mutations in BTM patients.8,31 Our study investigated for the first time the role of HFE H63D and C282Y mutations in the development of cardiac and hepatic hemosiderosis in Iranian patients with BTM. According to our results, the difference in genotype and allele distribution of HFE H63D mutation in patients with and without cardiac or hepatic hemosiderosis did not reach a significant level, indicating the futility of these mutations in conferring increased risk of cardiac and hepatic hemosiderosis. Similar results were reported by Turedi et al,13 in a recently published study of 33 Turkish patients with BTM. However, in our study, an elevated plasma ferritin level was found in the carriers of HFE H63D mutation, which indicated the modulating effects of the HFE H63D mutation on biochemical iron markers. Coinheritance of HFE H63D mutation may enhance the iron overload in patients with BTM, which may necessitate a more intensive iron chelation therapy in these patients to prevent hemosiderosis development. Our recent results were in accordance with the results of some previously published studies that indicated a positive association between HFE H63D mutation and serum ferritin levels.4,6,11 However, some other studies have not reported such an association.32,33 The possible reasons for these contradictory results may be related to variation in the study design (i.e., sample size, sample selection criteria) and the presence of gene-gene and gene-environment interactions in various studied populations.34 The present study has some limitations: (i) the cross-sectional design of the study limited the inclusion of control group, (ii) the other polymorphisms of HFE gene were not investigated, and (iii) the study population was relatively small.
Conclusions
Cardiac T2*MRI should be done for all patients with BTM regardless of their serum ferritin levels or hepatic T2*MRI values. HFE H63D and C282Y mutations are not major causes for development of cardiac and hepatic hemosiderosis in patients with BTM from the Zanjan province of Iran. However, a large-scale multi-center study in Iran is required to confirm these preliminary results.
Disclosure
The authors declared no conflicts of interest. The study was funded by a grant from Zanjan University of Medical Sciences (ZUMS), Deputy of Research and Technology (grant number A-12-104-1) Zanjan, Iran.
references
- 1. Mishra AK, Tiwari A. Iron overload in Beta thalassaemia major and intermedia patients. Maedica (Buchar) 2013 Sep;8(4):328-332.
- 2. Aessopos A, Farmakis D, Berdoukas V. Cardiac failure in β-thalassemia: diagnosis, prevention and management. Thalassemia Reports 2011 Dec;1(1):59-65.
- 3. Al-Khabori M, Bhandari S, Al-Huneini M, Al-Farsi K, Panjwani V, Daar S. Side effects of deferasirox iron chelation in patients with beta thalassemia major or intermedia. Oman Med J 2013 Mar;28(2):121-124.
- 4. Wilson MM, Al-Wakeel H, Said F, El-Ghamrawy M, Assaad M, El-Beshlawy A. Study of the effect of HFE gene mutations on iron overload in Egyptian thalassemia patients. Egypt J Med Hum Genet 2015 Apr;16(2):129-133.
- 5. Lebrón JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, et al. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell 1998 Apr;93(1):111-123.
- 6. Martins R, Picanço I, Fonseca A, Ferreira L, Rodrigues O, Coelho M, et al. The role of HFE mutations on iron metabolism in beta-thalassemia carriers. J Hum Genet 2004;49(12):651-655.
- 7. Madani HA, Afify RA, Abd El-Aal AA, Salama N, Ramy N. Role of HFE gene mutations on developing iron overload in beta-thalassaemia carriers in Egypt. East Mediterr Health J 2011 Jun;17(6):546-551.
- 8. Mokhtar DA, Hamdy MM, Badr AM. Frequency of human hemochromatosis (HFE) gene mutations in Egyptians with β-thalassemia. Egypt J Haematol 2013 Jan;38(1):36-40.
- 9. Agarwal S, Tewari D, Arya V, Moorchung N, Tripathi R, Chaudhuri G, et al. Status of HFE mutation in thalassemia syndromes in north India. Ann Hematol 2007 Jul;86(7):483-485.
- 10. Jowkar Z, Geramizadeh B, Shariat M. Frequency of two common HFE gene mutations (C282Y and H63D) in a group of Iranian Patients with cryptogenic cirrhosis. Hepat Mon 2011 Nov;11(11):887-889.
- 11. Enein AA, El Dessouky NA, Mohamed KS, Botros SK, Abd El Gawad MF, Hamdy M, et al. Frequency of hereditary hemochromatosis (HFE) gene mutations in Egyptian Beta thalassemia patients and its relation to iron overload. Open Access Maced J Med Sci 2016 Jun;4(2):226-231.
- 12. López-Escribano H, Ferragut JF, Parera MM, Guix P, Castro JA, Ramon MM, et al. Effect of co-inheritance of β-thalassemia and hemochromatosis mutations on iron overload. Hemoglobin 2012;36(1):85-92.
- 13. Turedi A, Oymak Y, Meşe T, Yaman Y, Bayraktaroglu S, Alpman A, et al. The effect of HFE polymorphisms on cardiac iron overload in patients with beta-thalassemia major. Pediatr Hematol Oncol 2013 Nov;30(8):755-760.
- 14. Unal S, Balta G, Gümrük F, Xu HG. Survey of Hfe gene C282Y mutation in Turkish Beta-Thalassemia patients and healthy population: a preliminary study. Turk J Haematol 2014 Sep;31(3):272-275.
- 15. Musallam KM, Cappellini MD, Wood JC, Motta I, Graziadei G, Tamim H, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with β thalassemia intermedia. Haematologica 2011 Nov;96(11):1605-1612.
- 16. Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program Book 2011 Dec 10;2011(1):443-450.
- 17. Youssef DM, Fawzy Mohammad F, Ahmed Fathy A, Aly Abdelbasset M. Assessment of hepatic and pancreatic iron overload in pediatric Beta-thalassemic major patients by t2* weighted gradient echo magnetic resonance imaging. ISRN Hematol 2013 Mar;2013:496985.
- 18. Taghizadeh Sarvestani R, Moradveisi B, Kompany F, Ghaderi E. Correlation between heart and liver iron levels measured by mri t2* and serum ferritin in patients with β-thalassemia major. Int J Pediatr 2016 Mar;4(3):1559-1567.
- 19. Yang G, Liu R, Peng P, Long L, Zhang X, Yang W, et al. How early can myocardial iron overload occur in beta thalassemia major? PLoS One 2014 Jan;9(1):e85379.
- 20. Majd Z, Haghpanah S, Ajami GH, Matin S, Namazi H, Bardestani M, et al. Serum ferritin levels correlation with heart and liver mri and lic in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J 2015 Apr;17(4):e24959.
- 21. Eghbali A, Taherahmadi H, Shahbazi M, Bagheri B, Ebrahimi L. Association between serum ferritin level, cardiac and hepatic T2-star MRI in patients with major β-thalassemia. Iran J Ped Hematol Oncol 2014;4(1):17-21.
- 22. Azarkeivan A, Hashemieh M, Shirkavand A, Sheibani K. Correlation between heart, liver and pancreas hemosiderosis measured by mri t2* among thalassemia major patients from Iran. Arch Iran Med 2016 Feb;19(2):96-100.
- 23. Gomes KB, Carvalho MG, Coelho FF, Rodrigues IF, Soares AL, Guimarães DA, et al. Lack of association of C282Y and H63D mutations in the hemochromatosis (HFE) gene with diabetes mellitus type 2 in a case-control study of women in Brazil. Genet Mol Res 2009 Oct;8(4):1285-1291.
- 24. Merchant R, Joshi A, Ahmed J, Krishnan P, Jankharia B. Evaluation of cardiac iron load by cardiac magnetic resonance in thalassemia. Indian Pediatr 2011 Sep;48(9):697-701.
- 25. Angulo IL, Covas DT, Carneiro AA, Baffa O, Junior JE, Vilela G. Determination of iron-overload in thalassemia by hepatic MRI and ferritin. Rev Bras Hematol Hemoter 2008 Dec;30(6):449-452.
- 26. Azarkeivan A, Hashemieh M, Akhlaghpoor S, Shirkavand A, Yaseri M, Sheibani K. Relation between serum ferritin and liver and heart MRI T2* in beta thalassaemia major patients. East Mediterr Health J 2013 Aug;19(8):727-732.
- 27. Chate SC, Manglani M, Pote M, Jankharia B. Assessment of cardiac iron overload in multiply transfused thalassemic children using T2* weighted cardiac magnetic resonance. Indian J Child Health 2015Dec;2(4):169-172.
- 28. Wu X, Jing Y, Pei F, Chen J, Feng X, He Y, et al. [Value of magnetic resonance imaging T2* tests in detecting heart and liver iron overload in patients with β-thalassemia major]. Nan Fang Yi Ke Da Xue Xue Bao 2013 Feb;33(2):249-252.
- 29. Parkes JG, Hussain RA, Olivieri NF, Templeton DM. Effects of iron loading on uptake, speciation, and chelation of iron in cultured myocardial cells. J Lab Clin Med 1993 Jul;122(1):36-47.
- 30. Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol 2010 Sep;56(13):1001-1012.
- 31. Oliveira TM, Souza FP, Jardim AC, Cordeiro JA, Pinho JR, Sitnik R, et al. HFE gene mutations in Brazilian thalassemic patients. Braz J Med Biol Res 2006 Dec;39(12):1575-1580.
- 32. Estevão IF, Peitl Junior P, Bonini-Domingos CR. Serum ferritin and transferrin saturation levels in β0 and β(+) thalassemia patients. Genet Mol Res 2011 Apr;10(2):632-639.
- 33. Mellouli F, El Borgi W, Kaabi H, Ben Hassen E, Sassi R, Hmida H, et al. [HFE gene mutations in Tunisian major beta-Thalassemia and iron overload]. Transfus Clin Biol 2006 Dec;13(6):353-357.
- 34. Gorroochurn P, Hodge SE, Heiman GA, Durner M, Greenberg DA. Non-replication of association studies: “pseudo-failures” to replicate? Genet Med 2007 Jun;9(6):325-331.