Acute lymphoblastic leukemia is the most common type of leukemia in young Omani patients comprising about 75% of all child acute leukemia in Oman. It is mainly of two subtypes: precursor B acute lymphoblastic leukemia (B-ALL), which represents 85% of Omani patients with acute lymphoblastic leukemia; and T-cell acute lymphoblastic leukemia (T-ALL) representing the remaining 15% of affected patients.1 T-ALL is the disease committed to any stage of T-cell lineage in which patients present with fever, enlarged thymus gland, bleeding, bruising, recurrent infections, tiredness for unknown reason, abdominal pain, and hyperkalemia related symptoms.2
Diagnostic approaches of the disease rely on the results of multiple laboratory investigations (i.e., immunophenotyping, cytogenetics, molecular biology) as well as the clinical picture of the disease. The two currently used techniques for immunophenotyping are flow cytometry and immunohistochemistry.3 Flow cytometry is a laser-based technique and is currently one of the most commonly used methods to differentiate and distinguish between the disease subtypes by recognizing the pattern of proteins expressed on the cell surface of lymphoid cells (immunophenotype characteristics of leukemic cells).4
The correct and rapid determination of the subtypes of acute lymphoblastic leukemia plays a crucial role in assessing the best risk oriented treatment for the patient to improve treatment outcomes.5 The flow cytometry results documented that all the patients included in the study were confirmed to have T-ALL owing to the presence of CD3 positivity (a pan cytomarker for T-lymphocytes). There are no previous papers in the literature that studied the characteristics of T-ALL in Omani patients. Hence, the full picture of the disease outcome and development is not completely understood in this group. We sought to study and classify the immunophenotype characteristics of Omani patients diagnosed with T-ALL and to correlate the results with age and gender as well as biological factors (peripheral and bone marrow cell blasts percentage) to understand the characteristics of the disease in Omani patients. Doing this will allow us to better predict disease outcome and determine an appropriate therapeutic plan.
Methods
We conducted a retrospective review of the records of patients with T-ALL diagnosed at our hospital. Ethical approval for the study was obtained from the Research and Ethics Committee of the College of Medicine and Health Sciences, Sultan Qaboos University. Relevant data necessary for the study was extracted from the patient’s case records obtained from the hospital information system database. All T-ALL patients who visited the Sultan Qaboos University Hospital from January 2002 to September 2015 were included in the study. Fifty patients who fulfilled the inclusion criteria were eligible for analysis, which consisted of Omani of both genders and all age categories. Expression of CD3 cytomarker as a pan cytomarker of T-cell lymphocytic lineage was mandatory for the diagnosis. Non-Omani and Omani patients with missing data regarding T-cell immunophenotype were excluded from the study. Other forms of missing data encountered such as insufficient results due to crushed bone marrow sample, insufficient sample to obtain results, and old data files that were not fully available in the system were all excluded in the final analysis.
Immunophenotypic characteristics were determined by identifying the expression of CD4 and CD8 markers on CD-3 bearing T-lymphoblasts. All patients were classified into three categories based on the type of antigen expression of CD4 and CD8 (i.e., immature T-cell (CD4-, CD8-), maturing T-cell (CD4+, CD8+), and mature T-cell (CD4-, CD8+ or CD4+, CD8-). The results were correlated with demographic factors of the patients (age and gender). Bone marrow lymphoblast and peripheral blood lymphoblast percentage were also correlated with the three patient groups.
All data were collected initially using a pre-designed Excel sheet and analyzed using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) Correlation was tested using ANOVA.
Results
Out of the 50 patients, only six were female (12.0%), and all these female patients presented with an immature T-ALL phenotype (pro T-ALL and pre T-ALL). Furthermore, only 21.0% of all patients with immature T-ALL subset were female. Overall, 56.0% of patients (n = 28) presented with immature T-ALL (pro T-ALL and pre T-ALL) phenotype, 24.0% (n = 12) with cortical phenotype and 20.0% (n = 10)
expressed features of mature (medullary) T-ALL phenotype [Table 1].
Table 1: Percentage of patients based on the differentiation stages of the neoplastic clone of T-ALL in correlation with gender.
|
Gender |
Male count, n
Total, % |
22
44.0 |
12
24.0 |
10
20.0 |
44
88.0 |
|
Female count, n
Total, % |
6
12.0 |
0
0.0 |
0
0.0 |
6
12.0 |
The percentage of patients under each developmental stage of leukemic T-cells is shown in Figure 1. The highest percentage of patients (56.0%) presented with an immature T-ALL phenotype (pro- and pre-T-ALL) (CD4-, CD8-), whereas 24.0% of cases expressed features of maturing T-ALL phenotype (cortical) (CD4+, CD8+). Only 20.0% of patients expressed mature (medullary) (CD4-, CD8+ or CD4+, CD8-) T-ALL phenotype. This was further classified into CD4-, CD8+ constituting 40.0% of patients and CD4+, CD8- constituting 60.0% of the subtype [Figure 2].
The average age of patients with immature T-ALL, maturing (cortical) T-ALL, and mature (medullary) T-ALL were 19.9, 20.5, and 17.3 years, respectively (p = 0.060). The average age for the total group of patients was 19.2 years [Figure 3]. The average bone marrow blast cell percentages were 77.7%, 88.6%, and 72.3% in the immature T-ALL, maturing T-ALL, and mature T-ALL groups, respectively, and was not statistically significant (p = 0.060). The average percentage of the bone marrow blasts for the whole group of patients was 79.5% [Figure 4].
The average peripheral blast cell percentages were 56.2%, 64.1%, and 79.6% in the immature T-ALL, maturing T-ALL, and mature T-ALL subtypes, respectively. We saw a rising trend but this did not reach statistical significance (p = 0.054). Overall, the average percentage of peripheral blood blasts of the whole group of patients was 66.6% [Figure 5].
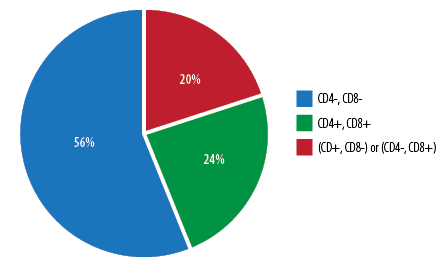
Figure 2: Distribution of patients with mature T-cell phenotype of T-cell acute lymphoblastic leukemia.
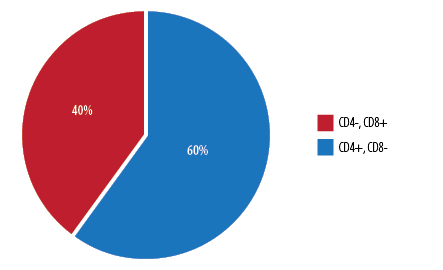
Figure 1: Distribution of patients based on differentiation stages of the neoplastic clone of T-cell acute lymphoblastic leukemia.
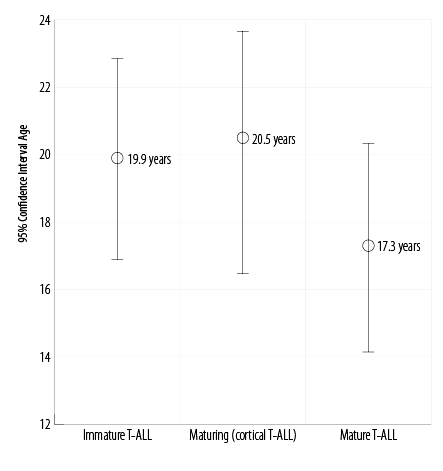
Figure 3: Correlation between average age and distinctive maturation stages of T-cell acute lymphoblastic leukemia subgroups (p = 0.060).
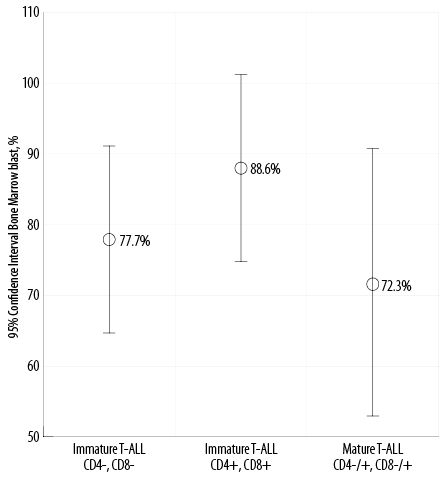
Figure 4: Correlation between bone marrow blast cells percentage and distinctive maturation stage of T-cell acute lymphoblastic leukemia (T-ALL) subgroups (p = 0.060).
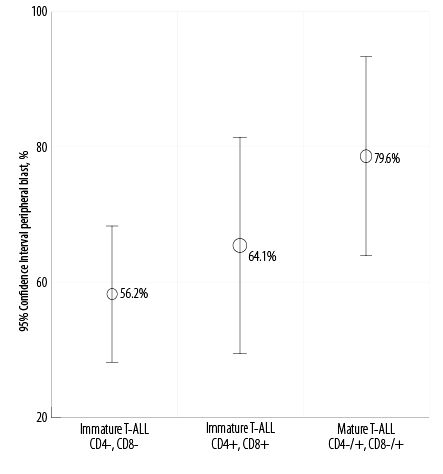
Figure 5: Correlation between peripheral blast cell percentage and distinctive maturation stage of T-cell acute lymphoblastic leukemia (T-ALL) subgroups
(p = 0.054).
Discussion
We initially aimed to study the full immunophenotypic characteristics of T-ALL in Omani patients including the disease subtypes and associated molecular markers of each subtype. Unfortunately, that could not be achieved because of the relative rarity of the reported cases at our institution and the incomplete data documentation of several patients in the electronic system. Hence, we relied on the three most important markers, namely CD4, CD8, and CD3 (surface and cytoplasmic), to study the immunophenotypic characteristics based on the European Group for the Immunological Characterization of Leukemia’s (EGIL) classification.6 The EGIL classification divides T-ALL into three developing stages based on the presence or absence of CD4 and CD8 markers. The immature phenotype (CD4-,CD8-) or double negative T-cell phenotype, maturing phenotype (CD4+, CD8+) also called the cortical or double positive phenotype, and the mature phenotype (CD4+, CD8- or CD8+, CD4-) also called medullary or single positive T-cell phenotype. The prevalence or absence of these markers was clearly documented in all patients analyzed in this study and CD3 was used to confirm the T-cell lineage as it is a pan T-cell marker.
We found that the immature T-ALL phenotype was most commonly seen in Omani patients, both male and female. On the other hand, the cortical phenotype was the second most common phenotype found in our patients while the mature phenotype was the least common. Our results were in concordance with other studies from the Middle East and Mediterranean.1,7 A recent study by Lahjouji et al,7 and two other older studies from Italy and France also showed similar results.8,9
The importance of understanding the T-ALL characteristics in a given population arises from the prognostic probability associated with some subtypes of the disease. For T-ALL, previous literature show that patients who were identified to have the cortical T-ALL phenotype were most likely to have better prognostic outcomes. Ongoing research and efforts have identified additional markers and molecular factors associated with cortical T-ALL phenotype that led to this prognostic effect, which may improve the therapeutic plan of the disease.10,11 However, no prognostic association was found with medullary and immature T-ALL phenotype.
We had a male-to-female ratio of 7:1 in our patient cohort. Gender was highly correlated with disease incidence, which was significantly more prevalent in males than females (p = 0.007). Similar results were found in Moroccan patients in a study by Lahjouji et al,7 (p = 0.033) as well as one by Pui et al.12
Furthermore, an important highlight of this study was that 100% of Omani female with T-ALL presented with the immature phenotype. However, previous studies7,12 did not show a proportional difference in the female distribution under the sub-classification of the disease as noted with
our patients.
Our study did not evaluate any prognostic differences, clinical features, or drug pharmacokinetics between males and females. However, a prospective study done in the USA in 2005 concluded that females have better prognosis than males.13
We also studied the age of Omani patients with T-ALL and correlated the average age of patients with each disease subtype. However, there was no significant correlation between the average age of patients and disease subtype (p = 0.060). The average age of the total group of patients was 19.2 years while the average age of patients with immature, maturing, and mature phenotypes were 19.9, 20.5, and 17.3 years, respectively. Furthermore, no significant variation was observed in the average age of males and females, which is in agreement with the results of previous studies.7,14
We also evaluated the peripheral blood blast cell percentage as well as bone marrow blast cell percentage. However, in 12.0% of these patients, due to improper documentation, we were unable to obtain their complete results. In the remaining patients (n = 44) studied, all presented with a bone marrow blasts cells percentage more than 20.0% at the first visit. Only 22.7% of patients presented on their first visit with a peripheral blood blast cells percentage less than 20.0%. These results are in agreement with a study from the USA that clarified that peripheral blood blast cells cannot always be efficient and useful in making the disease diagnosis.15 However, the same is not applied for bone marrow blast cells percentage, which is always high and diagnostically useful.
On comparing the average percentage of bone marrow blast cells in each disease subtypes, no significant differences were found and no association was noticed between the bone marrow blast cells percentage and the three subtypes of T-ALL
(p = 0.060). However, the percentage of bone marrow blast cells in patients with cortical T-ALL was slightly higher than the other two subtypes of the disease. Furthermore, no association was found between the subtypes of T-ALL and peripheral blood blast cells percentage (p = 0.054). Nonetheless, medullary T-ALL showed a slightly higher percentage of peripheral blood blasts than the other subtypes of the disease.
A recent review summarized T-ALL as an aggressive malignancy caused due to an accumulation of several genetic lesions that hamper the development of T-cells.16 Although there are emerging technological advances to screen new mutations, we did not recognize in the literature any study correlating the different subtypes of T-ALL and bone marrow blast cells percentage.
Conclusion
This study is the first to report the subtypes of T-ALL in Oman in the English language literature. It is hoped that this will lead to a better understanding of the disease outcomes. Our results were in agreement with previously published results from the Middle East and the Mediterranean area countries such as France, Italy, and Morocco.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. al Lamki Z, Wali YA, Shah WM, Zachariah M. Relapsed acute leukemia in children: Oman experience. Pediatr Hematol Oncol 2004 Mar;21(2):167-173.
- 2. Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009 Feb;27(6):911-918.
- 3. Falini, B. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood 2006;108(6):1999-2005.
- 4. Kresno SB, Gondhowiardjo S. Disregulation process of cancer cell proliferation. Acta Med Indones 2004 Jul-Sep;36(3):169-176.
- 5. Bhojwani D, Howard SC, Pui CH. High-risk childhood acute lymphoblastic leukemia. Clin Lymphoma Myeloma 2009;9(Suppl 3):S222-S230.
- 6. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al; European Group for the Immunological Characterization of Leukemias (EGIL). Proposals for the immunological classification of acute leukemias. Leukemia 1995 Oct;9(10):1783-1786.
- 7. Lahjouji A, Bachir F, Bennani S, Quessar A, Amzazi S. The immunophenotype of adult T acute lymphoblastic leukemia in Morocco. Exp Oncol 2015 Mar;37(1):64-69.
- 8. Foà R, Baldini L, Cattoretti G, Foa P, Gobbi M, Lauria F, et al. Multimarker phenotypic characterization of adult and childhood acute lymphoblastic leukaemia: an Italian multicentre study. Br J Haematol 1985 Oct;61(2):251-259.
- 9. Boucheix C, David B, Sebban C, Racadot E, Bené MC, Bernard A, et al; French Group on Therapy for Adult Acute Lymphoblastic Leukemia. Immunophenotype of adult acute lymphoblastic leukemia, clinical parameters, and outcome: an analysis of a prospective trial including 562 tested patients (LALA87). Blood 1994 Sep;84(5):1603-1612.
- 10. Shiozawa Y, Takita J, Kato M, Sotomatsu M, Koh K, Ida K, et al. Prognostic significance of leukopenia in childhood acute lymphoblastic leukemia. Oncol Lett 2014 Apr;7(4):1169-1174.
- 11. Taskov H, Dimitrova E, Serbinova M, Mendisova L, Bobev D. Immunological subtypes of childhood acute lymphoblastic leukemia in Bulgaria. Leuk Res 1995 Nov;19(11):877-881.
- 12. Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 2010 Feb;24(2):371-382.
- 13. Pui CH, Pei D, Sandlund JT, Campana D, Ribeiro RC, Razzouk BI, et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005 Nov;23(31):7936-7941.
- 14. Möricke A, Zimmermann M, Reiter A, Gadner H, Odenwald E, Harbott J, et al. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr 2005 Nov-Dec;217(6):310-320.
- 15. Weinkauff R, Estey EH, Starostik P, Hayes K, Huh YO, Hirsch-Ginsberg C, et al. Use of peripheral blood blasts vs bone marrow blasts for diagnosis of acute leukemia. Am J Clin Pathol 1999 Jun;111(6):733-740.
- 16. Girardi T, Vicente C, Cools J, De Keersmaecker K. The genetics and molecular biology of T-ALL. Blood 2017 Mar;129(9):1113-1123.