Stevens-Johnson syndrome (SJS) is a rare and acute life-threatening condition which is almost always precipitated by drugs.1 The disease has an immunological pathogenesis, and it is clinically characterized by severe mucocutaneous reactions resulting in epidermal necrosis and mucosal erosions.2 It generally manifests as fever and influenza-like symptoms. Skin lesions occur one to three days after the onset of these symptoms. Supportive care in a well-equipped unit is the mainstay in the management of SJS. After the identification and elimination of all possible causative drugs, management should include aggressive fluid and electrolyte replacement, nutritional support, and use of topical antiseptic preparations with surveillance for secondary infections.3 Death may occur mostly due to sepsis and a mortality rate of more than 10% at six weeks was reported.4
Mucocutaneous lesions usually heal without sequelae. However, some patients may experience mucosal scarring, which may cause severe long-term morbidities including airway compromise, gastrointestinal passage disorders, and ocular surface damage with the potential to lead to blindness.5,6 Genital mucositis in female patients may also be an important cause of long-term morbidity secondary to mucosal scarring.6 Nevertheless, vaginal synechiae with complete obliteration of the vaginal canal is rare, and surgery may be required to treat hematocolpos and recover sexual activity.7
In this report, we present a case with distal vaginal synechiae necessitating a surgical approach, which occurred after an episode of SJS. We also reviewed the literature to reveal cases that required surgical management for long-term genital sequelae and discuss the preventive measures.
Case Report
A family physician referred a 33-year-old nulligravid woman to our department for vaginal synechiae after a failed attempt to remove her levonorgestrel-releasing intrauterine device (IUD). The IUD was inserted for heavy menstrual bleeding five years ago and the patient was amenorrheic for the last three years. She was divorced three years ago and had no coital activity since.
Her medical history revealed that she had a diagnosis of SJS 10 months ago. She had been prescribed ciprofloxacin for urinary tract infection 10 days before the onset of her initial flu-like symptoms and high fever. Initially, she was treated with non-steroidal anti-inflammatory drugs. However, diffuse erythema, target lesions, blisters, and painful hemorrhagic erosions had appeared on her face, trunk, arms, and legs three days following initial symptoms. Diffuse hemorrhagic crusting bullous lesions had been seen [Figure 1]. After hospital admission, the clinical diagnosis of SJS was confirmed by a skin biopsy. Intensive care management was provided and an antiseptic paraffin impregnated gauze dressing containing 0.5 g chlorhexidine acetate per 100 g (BACTIGRAS)® was applied to the eroded areas. The patient was discharged from the hospital after 46 days with almost complete skin re-epithelization. Unfortunately, severe eye complications (xerophthalmia and bilateral blepharophimosis) developed.
On pelvic examination, she had complete vaginal obliteration beginning at the level of the introitus. A pelvic ultrasound showed normal ovaries and uterus with a properly located IUD. After obtaining her informed consent for surgical vaginal reconstruction and removal of the IUD, we performed an examination under general anesthesia, which confirmed complete vaginal synechiae. All attempts to separate the fused vaginal walls either with blunt or sharp dissection failed. Therefore, following a transverse incision on posterior fourchette, we performed dissection in the upward direction between posterior vaginal wall and rectum. Dissection was extended up to the level of the uterine cervix, and peritoneal cavity was entered through the pouch of Douglas. We grasped the cervix with a tenaculum and made a colpotomy on the posterior wall of the proximal vagina at the level of posterior fornix. Purulent fluid filling the vagina was drained. The upper half of the vagina and cervix was spared [Figure 2], and the healthy vaginal tissue was 4–5 cm in length. After the removal of IUD, we pulled the healthy proximal vagina downwards and sutured it to the introitus. A vagina with a depth of 5 cm developed. We completed the surgery after inserting a povidone-iodine impregnated sponge into the vagina. Her postoperative course was uneventful. We removed the vaginal sponge on the first postoperative day and discharged the patient on the following day. We instructed the patient to refrain from coitus and vaginal medications for six weeks. She was re-examined six weeks after the surgery. On follow-up, she had a regular menstrual cycle with approximately 5 cm healthy vaginal tissue.
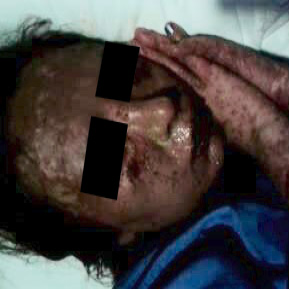
Figure 1: Blisters and painful hemorrhagic erosions on the skin.
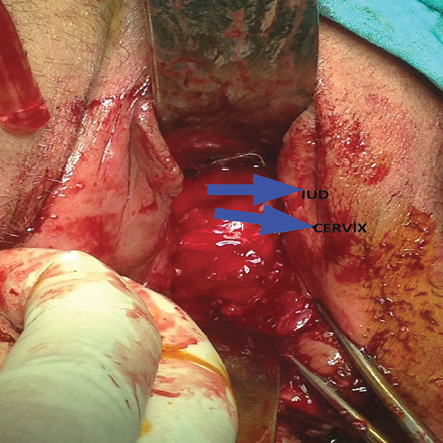
Figure 2: There was no occlusion at the proximal vagina.
Discussion
Vulvovaginal involvement is as common as 70% during the acute phase of SJS.7 The most frequently detected genital lesions are erosive and ulcerative vaginitis and vulvar bullae. These lesions tend to resolve in 1–8 weeks, but they may persist for several months and up to a year following the acute period of the disease. Long-term consequences of genital involvement are labial agglutination, introital stenosis, vaginal synechiae and stenosis, vaginal and vulval adenosis, hematocolpos, and hematometra. Endometriosis may occur due to scarring in up to 28% of patients.8,9
The main complaints secondary to these long-term complications are dryness, itching, burning sensation, post-coital bleeding, dyspareunia, or the inability to have sexual intercourse.9 Nevertheless, complete vaginal obliteration is rare according to the review of the published literature. In the largest series published on this issue, Meneux et al,7 reported that although genital lesions are common during the acute phase of the disease, sequelae occurred in only five of 40 patients (12.5%). In that group, two of the five women had surgical correction with long-term genital consequences. Among those, one had a recurrence six months post-surgery, and the other had no recurrence, but she was unable to have sexual intercourse.7 Graham-Brown et al,10 reported another case who developed hematocolpos due to complete vaginal stenosis following SJS in pregnancy. Wilson et al,11 described a case with severe vaginal stenosis who underwent a successful surgical correction. Hart et al,12 reported vaginoscopic division of vaginal adhesions for an 11-year-old girl after recovery from the systemic phase of SJS and they recommended that all patients with SJS with vaginal involvement undergo routine follow-up to evaluate and treat vaginal adhesions. In another report, a girl who developed SJS as a child experienced hematometra after the onset of puberty, and she required surgery to treat genital adhesions.13 Murphy and Brant,14 published a case with severe vaginal adhesions that led to the fusion of introitus. The patient was a 14-year-old girl who presented with lower abdominal pain and amenorrhea that persisted for five months. She was diagnosed with hematocolpos secondary to SJS and underwent surgery for fused introitus.14 Similarly, a 10-year-old girl with a history of SJS at age 4 was diagnosed with hydrometrocolpos secondary to extensive labial synechiae that completely covered the clitoris, urethral meatus, and vaginal opening. She required surgical reconstruction.15 Madhuri et al,16 reported a 32-year-old patient with a history of SJS who underwent excision of a single thick adhesion across the entire upper-third of the vagina.
In our patient, we detected complete synechiae of the distal vagina beginning at the level of the introitus. Since she was amenorrheic for the last three years due to the levonorgestrel-releasing IUD, hematocolpos, and hematometra did not develop. Additionally, she did not report coital problems because she was not sexually active. Her family physician incidentally diagnosed vaginal obliteration after a failed attempt to remove her IUD. Otherwise, she did not report any genital complaints. The surgery revealed that she had pyocolpos occupying the upper half of the vagina. Her vaginal synechiae was so firm that separation of the fused vaginal walls either with blunt or sharp dissection was impossible. Therefore, rectovaginal space dissection became inevitable to treat the obstruction.
For female patients with SJS, genital examination should be scheduled as early as possible to avoid genital tract obstruction and future sexual problems.15 This is especially mandatory in patients who report vaginal discomfort or discharge since these complaints indicate the presence of vaginal erosion and ulceration. In this situation, early identification may be possible, and early management may prevent the development of vulvovaginal adhesions and associated subsequent complications.16 Placing a soft mold in the vagina with the use of local corticosteroids until resolution of the acute phase of illness may be considered to prevent adhesions.8 However, such an approach poses a risk of infection and sepsis in the acute phase due to systemic absorption of topical steroids.9 Instead, a lubricant gel can be applied to lesions in the acute phase to prevent adhesions. Also, sexual intercourse immediately after the acute phase following wound epithelization was proposed by some authors to help reduce stenosis, but most patients may reject this.6,8 When fine adhesions are detected early in the course of SJS, the division of these adhesions as a relatively minor procedure may be helpful in preventing the development of thick fibrous bands and severe sequelae such as introital stenosis, vaginal narrowing, or complete vaginal canal obliteration.14 Gynecology consultation was not scheduled for our patient during or immediately after the acute phase of SJS. This might have contributed to her significant vaginal sequelae, which was detected incidentally since she was sexually inactive and amenorrheic.
Conclusion
Genital involvement is frequent in the acute phase of SJS, but long-term consequences are not so uncommon and symptomatic genital tract obstruction is rarely seen. On the other hand, such an obstruction usually necessitates major surgical procedures. The review of the literature showed only eight cases who required surgery for long-term genital sequelae. Therefore, the gynecologists should examine women with SJS as early as possible during the acute phase of condition since preventive measures are available that may help reduce the risk of severe long-term sequelae.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993 Jan;129(1):92-96.
- 2. Villada G, Roujeau JC, Clérici T, Bourgault I, Revuz J. Immunopathology of toxic epidermal necrolysis. Keratinocytes, HLA-DR expression, Langerhans cells, and mononuclear cells: an immunopathologic study of five cases. Arch Dermatol 1992 Jan;128(1):50-53.
- 3. Mukasa Y, Craven N. Management of toxic epidermal necrolysis and related syndromes. Postgrad Med J 2008 Feb;84(988):60-65.
- 4. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al; RegiSCAR study group. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013 May;133(5):1197-1204.
- 5. Roujeau JC, Chosidow O, Saiag P, Guillaume JC. Toxic epidermal necrolysis (Lyell syndrome). J Am Acad Dermatol 1990 Dec;23(6 Pt 1):1039-1058.
- 6. Letko E, Papaliodis DN, Papaliodis GN, Daoud YJ, Ahmed AR, Foster CS. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol 2005;94(4):419-436.
- 7. Meneux E, Paniel BJ, Pouget F, Revuz J, Roujeau JC, Wolkenstein P. Vulvovaginal sequelae in toxic epidermal necrolysis. J Reprod Med 1997 Mar;42(3):153-156.
- 8. Kaser DJ, Reichman DE, Laufer MR. Prevention of vulvovaginal sequelae in stevens-johnson syndrome and toxic epidermal necrolysis. Rev Obstet Gynecol 2011;4(2):81-85.
- 9. Niemeijer IC, van Praag MC, van Gemund N. Relevance and consequences of erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in gynecology. Arch Gynecol Obstet 2009 Nov;280(5):851-854.
- 10. Graham-Brown RA, Cochrane GW, Swinhoe JR, Sarkany I, Epsztejn LJ. Vaginal stenosis due to bullous erythema multiforme (Stevens-Johnson syndrome). Case report. Br J Obstet Gynaecol 1981 Nov;88(11):1156-1157.
- 11. Wilson EE, Malinak LR. Vulvovaginal sequelae of Stevens-Johnson syndrome and their management. Obstet Gynecol 1988 Mar;71(3 Pt 2):478-480.
- 12. Hart R, Minto C, Creighton S. Vaginal adhesions caused by Stevens-Johnson syndrome. J Pediatr Adolesc Gynecol 2002 Jun;15(3):151-152.
- 13. Pliskow S. Severe gynecologic sequelae of Stevens-Johnson syndrome and toxic epidermal necrolysis caused by ibuprofen: a case report. J Reprod Med 2013 Jul-Aug;58(7-8):354-356.
- 14. Murphy MI, Brant WE. Hematocolpos caused by genital bullous lesions in a patient with Stevens-Johnson syndrome. J Clin Ultrasound 1998 Jan;26(1):52-54.
- 15. de Jesus LE, Dekermacher S, Manhães CR, Faria LM, Barros ML. Acquired labial sinechiae and hydrocolpos secondary to Stevens-Johnson syndrome. Urology 2012 Oct;80(4):919-921.
- 16. Madhuri TK, Kremer C. Early gynaecological assessment following Stevens-Johnson syndrome/toxic epidermal necrolysis. J Obstet Gynaecol 2010;30(8):871-872.