|
Introduction
Monoclonal immunoglobulin deposition diseases (MIDD) include primary and light chain deposition disease. These entities are essentially of similar pathologic processes characterized by the deposition of immunoglobulins in soft tissues and viscera compromising their function. The accumulation of immunoglobulin molecules occurs before the development of the plasma cell neoplasm/lymphoplasmacytic neoplasm that is typically associated with MIDD. The above two processes differ in the chemical nature and properties of the deposited substance. The light chains of primary amyloidosis are predominantly lambda, in contrast to the kappa light chains seen in LCDD. The former shows affinity for Congo red stain and appears fibrillary under electron microscope; whereas, the latter do not possess the beta-pleated configuration typical of amyloid and therefore do not stain with these dyes. The most common manifestation of LCDD is renal involvement presenting with nephrotic syndrome and renal failure.1 Liver failure has rarely been reported in LCDD.2
Case Report
A 66 year-old male presented with severe jaundice and pruritus. There was no history of drug intake and physical examination revealed a hard, nodular liver with a span of 20 cm below the costal margin. The spleen was not palpable. Liver function tests showed the following results: total bilirubin: 11.3 mg/dl; direct bilirubin: 8.1 mg/dl; AST: 177 U/L (normal: 5-40 U/L); ALT: 75 U/L (N: 5-40 U/L); alkaline phosphatase: 1965 U/L (N: 96-279 U/L); GGT: 458 U/L (N: <50); and serum albumin: 2.7 g/dl (N: 3.7-5.3). LDH and α-feto protein were within normal limits. Serological markers for hepatitis A, B, C, E and HIV, as well as ANA were negative. Serum creatinine was normal and ultrasound of the abdomen showed hepatomegaly with pericholecystic echogenecities. There was no evidence of any space occupying lesion or biliary obstruction. CT abdomen revealed hepatomegaly, but no periportal lymph nodes, minimal bowel wall thickening in the descending colon, rectum and sigmoid were noted. Endoscopy revealed evidence of atrophic gastritis, loss of vascularity, edema and granularity in the rectum. Rectal biopsies to rule out ulcerative colitis showed mild focal inflammation. Considering the clinical and laboratory investigations; granulomatous, immunologic and infiltrative etiologic possibilities were taken into account.
Liver biopsy showed hepatic tissue with abundant homogeneous eosinophilic deposits in the lobules and portal tracts. Hepatocytes, bile ducts and ductules were compressed by the perisinusoidal deposits and hepatocytes appeared atrophic. Ductular and canalicular bile plugs were present. Sinusoids had dilated thickened walls; however, there were no abnormal lymphoid or plasma cell infiltrates. The amorphous pink deposits were Congo red negative, PAS positive and stained negatively for collagen. Immunostaining for light chains gave equivocal results. Based on the morphology on routine staining, the non-Congophilic nature of the deposits and the strong PAS staining of sinusoidal walls; diagnosis of Light Chain Deposition Disease (LCDD) was suggested. Other possibilities considered were heavy chain deposition disease and a mixed light and heavy chain deposition disease. Urinalysis for Bence Jones proteins, serum electrophoresis and bone marrow aspiration were suggested. The patient’s urine was found to be positive for Bence Jones proteins and M band was demonstrable in the serum electrophoresis. The bone marrow aspirate showed plasmacytosis (33%). The background of the marrow showed eosinophilic amorphous material, as was observed in the liver with similar staining properties. Therefore, a diagnosis of LCDD of the liver and bone marrow with plasma cell dyscrasia was made. The patient’s condition deteriorated with worsening of liver functions. He was discharged and lost to follow-up.
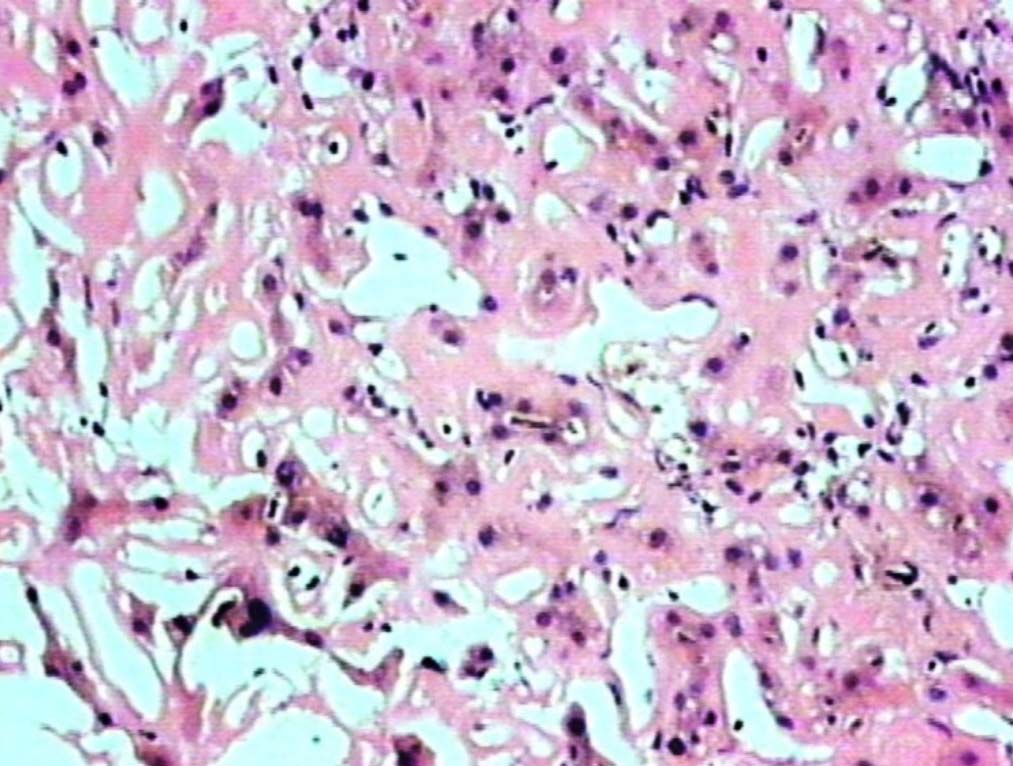
Figure 1: Liver showing homogenous, eosinophilic deposits, atrophic cords of hepatocytes and prominent sinusoids (H&E, original magnification × 200).
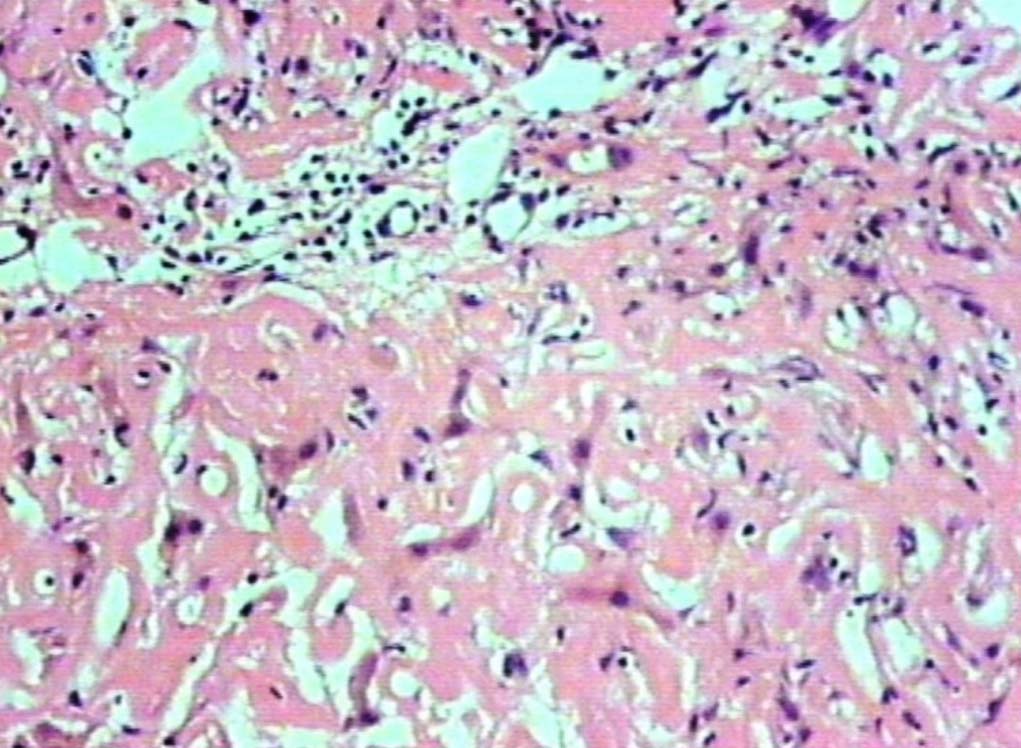
Figure 2: Deposits in the portal areas and in the lobules (H&E, original magnification × 200).

Figure 3: Bile plugs in canaliculi (H&E, original magnification × 400).
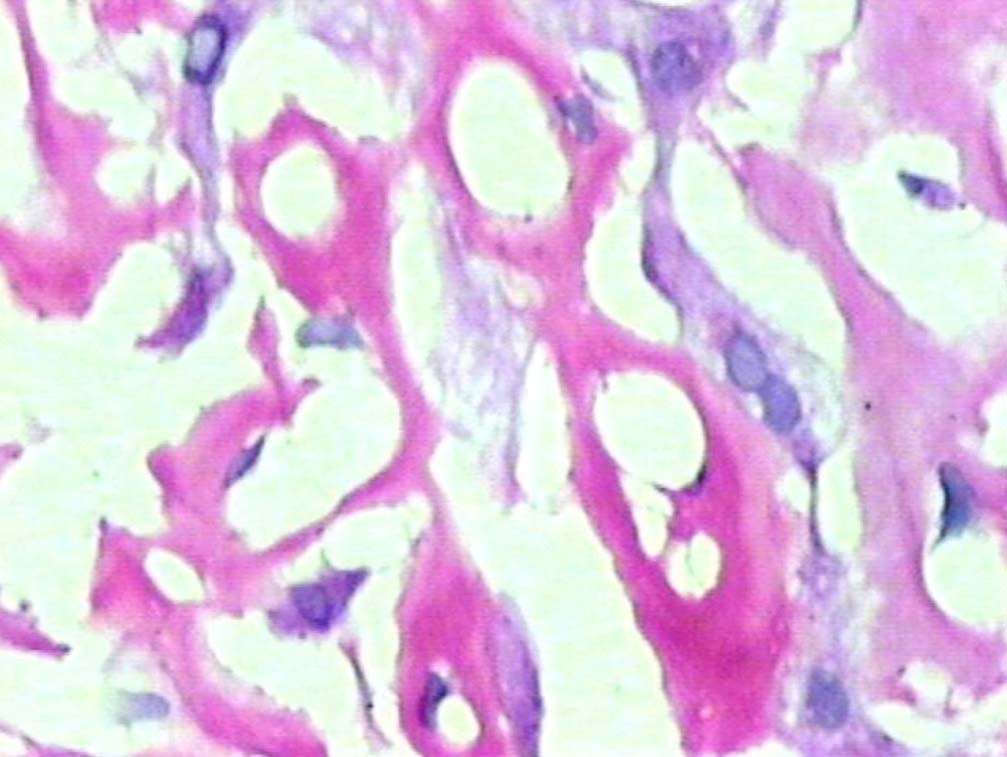
Figure 4: PAS positive deposits along sinusoids (original magnification × 400).
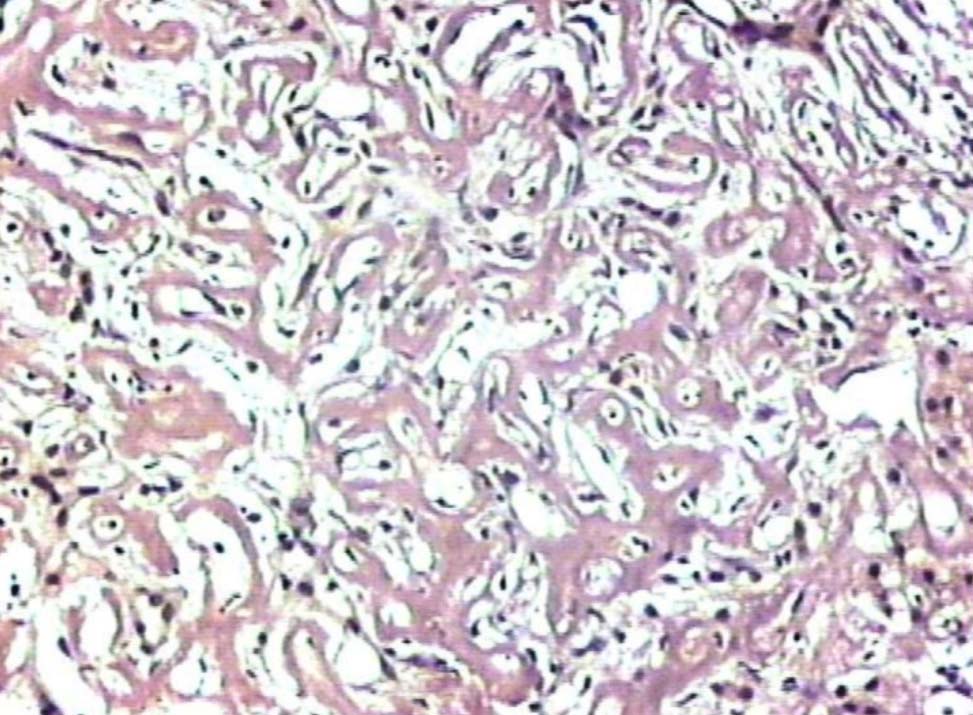
Figure 5: Liver showing deposits negative for Congo red stain (original magnification × 200).
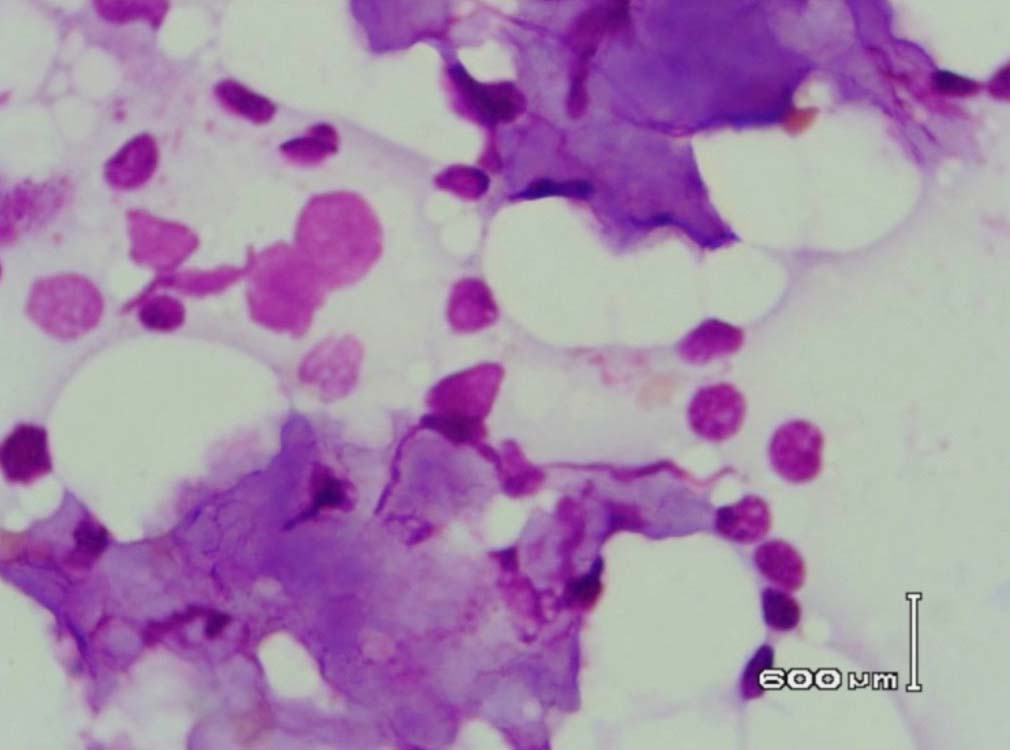
Figure 6: Bone marrow aspirate showing background of eosinophilic amorphous material (Leishman stain, original magnification × 400).
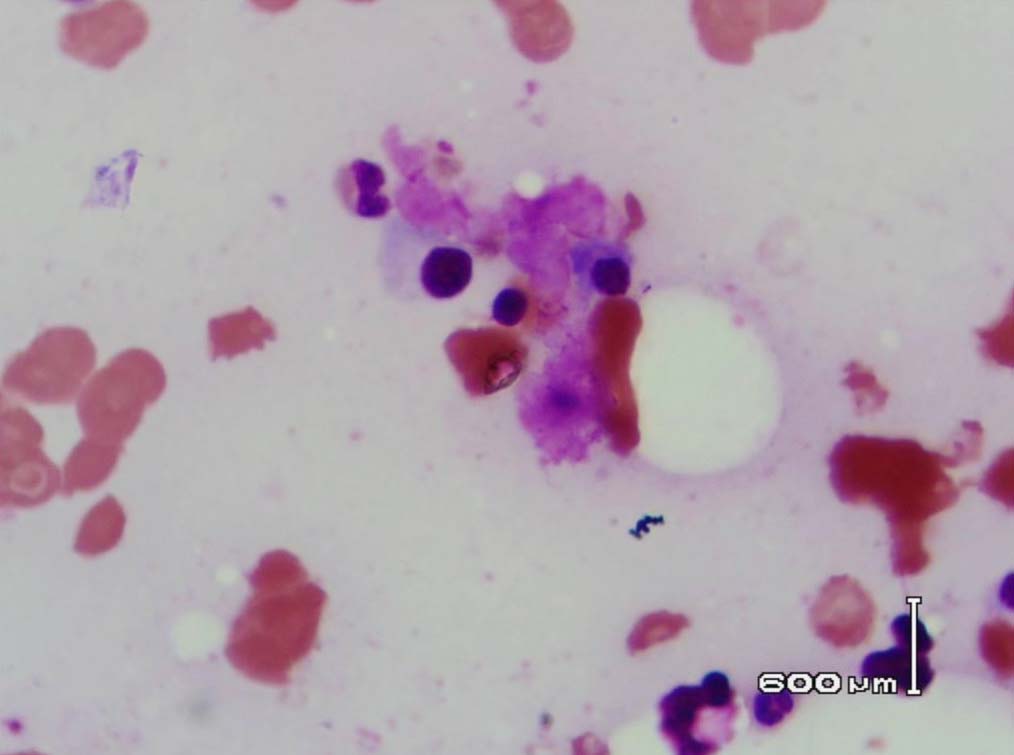
Figure 7: Bone marrow showing plasma cells surrounded by the eosinophilic amorphous material (Leishman stain, original magnification × 400).
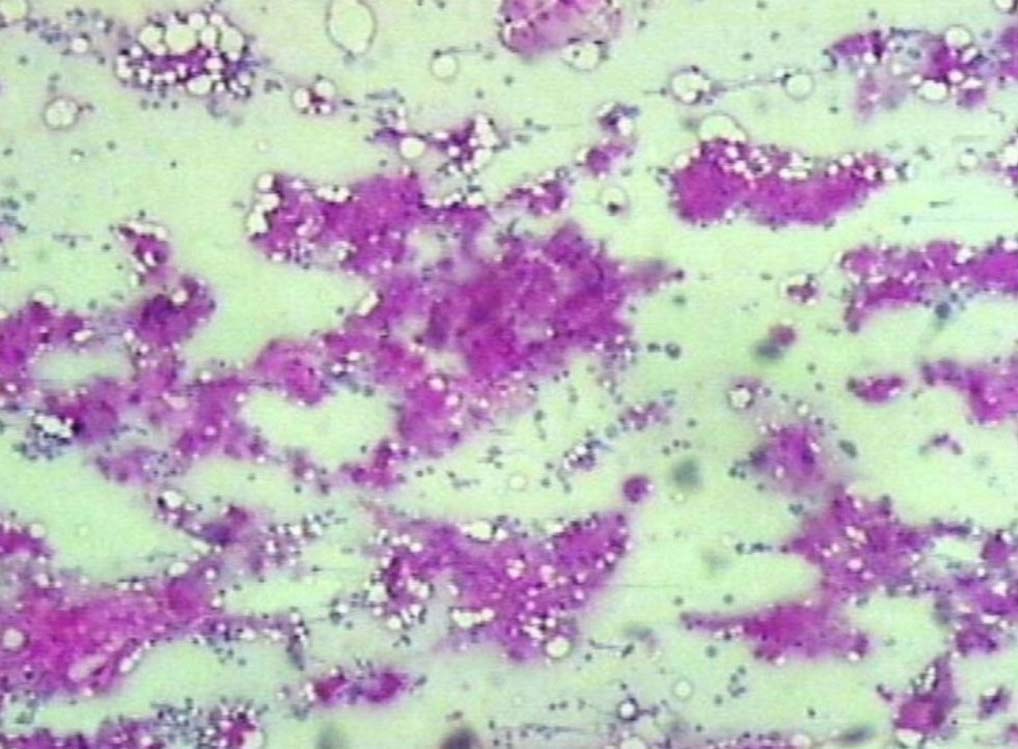
Figure 8: PAS positive deposits in bone marrow (original magnification × 200).
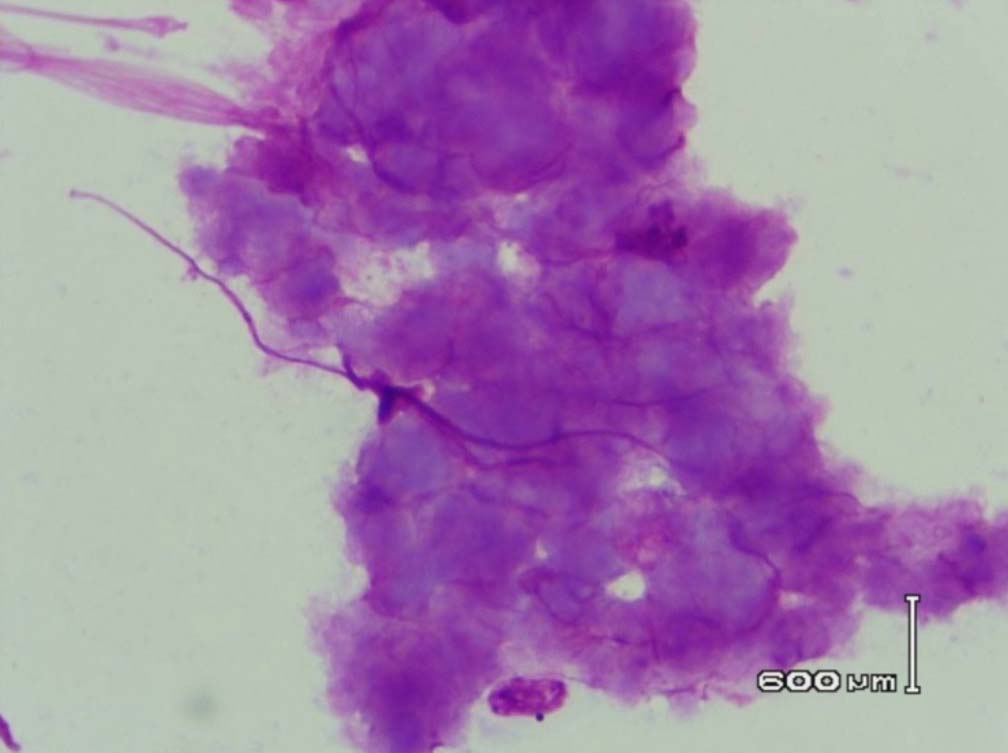
Figure 9: Amyloid like material with a Cumulus cloud like appearance in the bone marrow (PAS stain, original magnification × 400).
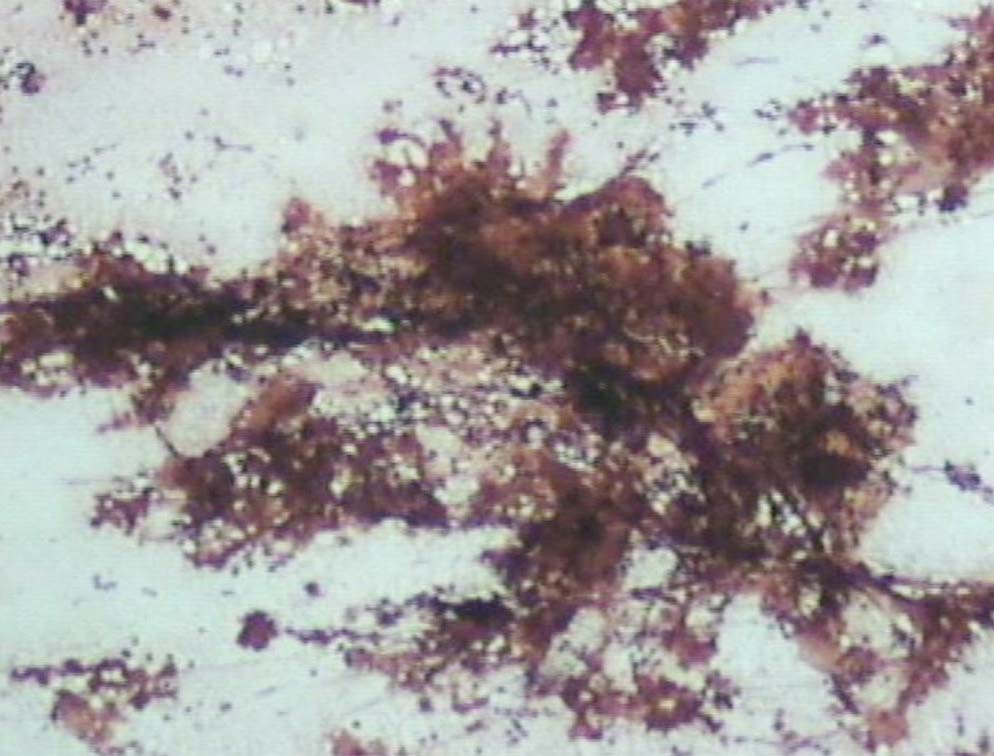
Figure 10: The deposits are Congo red negative (original magnification × 200).
Discussion
LCDD is a multisystem disease usually associated with plasma cell dyscrasias or multiple myeloma. It is characterized by the deposition of monoclonal, amorphous, noncongophilic light chains in multiple organs leading to compromised organ function.
Renal involvement is seen very commonly in LCDD, presenting as proteinuria, nephrotic syndrome or renal insufficiency. The liver is usually involved in association with renal lesions. Isolated liver involvement has been reported so far in only five cases of LCDD; two of these patients were diagnosed to have multiple myeloma and developed LCDD after the onset of treatment.3,4
The current reported patient presented with massive hepatomegaly, severe cholestasis, markedly deranged liver functions and normal renal functions. Cases with large hepatomegaly, disturbance of LFT, cholestasis and portal hypertension have only been reported occasionally.2,5,6 Four other reported cases of isolated liver LCDD and patients with both liver and renal involvement showed either mild liver enlargement and/only mild to moderate abnormalities in LFT.1,6
Liver involvement in LCDD is usually in the form of deposits over the basement membranes of the biliary tracts and sinusoids, and hepatic parenchyma is usually spared.1 Liver biopsy in our case showed extensive homogeneous eosinophilic deposits in the lobules and portal areas. The hepatocytes were compressed by the perisinusoidal deposits. Bile plugs were noted in the bile ducts and the canaliculi. However, unlike in the case reported by Faa et al.2 there were no bile infarcts. Cholestasis probably results from the compression of the bile ducts by the portal deposits. Three of the cases with isolated liver involvement previously reported showed deposition of non-amyloid light chains as well as amyloid.2,7,8 Our patient did not show evidence of amyloid deposition.
Leishman stained bone marrow in our patient showed plasmacytosis and extensive deposition of eosinophilic amorphous PAS positive and Congo Red negative “amyloid-like” material. The deposits had a “cumulus cloud” like effect, with an almost transparent center, dense and opaque at the periphery. The presence of light chains in the bone marrow observed in this case is unique and deserves mention. Such deposits have been reported in a patient with amyloidosis.9 Review of the literature shows that this feature has not been described in any of the LCDD cases reported so far.
Conclusion
Myeloma is the underlying disease in two thirds of patients with LCDD. However, it can also occur in the absence of any detectable hematological disorder when it is called idiopathic LCDD. Our patient presented with hepatomegaly and jaundice and the work up of this revealed an underlying myeloma. To our knowledge, there are only two other cases in the literature in which LCDD of the liver without renal involvement was the first manifestation of plasma cell dyscrasia.7,8 The clonal proliferations of plasma cells produce an immunoglobulin molecule that accumulates in tissues prior to the development of a large tumor burden.
Acknowledgements
Authors reported no conflict of interest and no funding was received for this work.
References
1. Samanez C, Domingo A, Cibeira MT, Miquel R, Soler M, Bladé J. Development of rapidly progressive liver light chain deposition under VAD chemotherapy in multiple myeloma. Eur J Haematol 2006 Jan;76(1):83-85.
2. Faa G, Van Eyken P, De Vos R, Fevery J, Van Damme B, De Groote J, et al. Light chain deposition disease of the liver associated with AL-type amyloidosis and severe cholestasis. J Hepatol 1991 Jan;12(1):75-82.
3. Weisel KC, Böckeler M, Bianchi L, Terracciano LM, Mayer F, Kanz L. Development of rapid light-chain deposition disease in hepatic arteries with severe ischemic cholangitis in a multiple myeloma patient treated with melphalan, prednisone and lenalidomide. Int J Hematol 2009 Jan;89(1):91-94.
4. Michopoulos S, Petraki K, Petraki C, Dimopoulos MA. Light chain deposition disease of the liver without renal involvement in a patient with multiple myeloma related to liver failure and rapid fatal outcome. Dig Dis Sci 2002 Apr;47(4):730-734.
5. Nath SV, Peiris M, Bishton MJ, Maxwell E, Prince HM. Light chain deposition disease presenting as massive hepatomegaly. Pathology 2010 Apr;42(3):307-310.
6. Pelletier G, Fabre M, Attali P, Ladouch-Badre A, Ink O, Martin E, et al. Light chain deposition disease presenting with hepatomegaly: an association with amyloid-like fibrils. Postgrad Med J 1988 Oct;64(756):804-808.
7. Casiraghi A, De Paoli A, Assi A, Palladini G, Lavazza MT, Beretta A, et al. Hepatic amyloidosis with light chain deposition disease - a rare association.Dig Liver Dis,2000; 32:795-798.
8. Gireli C, Giovanni L, Rocca F. Light chain deposition disease of the liver. Eur J Gastroenterol Hepatol 1999;10:429-430 .
9. Conn RB Jr, Sundberg RD, Sundberg D. Amyloid disease of the bone marrow. Diagnosis by sternal marrow a spiration. Am J Pathol 1961 Jan;38:61-71.
|