Pneumothorax in newborns has a significant mortality and morbidity. It occurs more frequently in the first three days of the neonatal period than at any other time of life, and demands prompt management.1–3 The rate of occurrence can increase up to 30% in patients who have an underlying lung disease (e.g., meconium aspiration syndrome (MAS)) or who need mechanical ventilation at birth.1 Other factors such as continuous positive airway pressure and positive pressure ventilation further increase the incidence of pneumothorax.1,2
The early diagnosis and treatment of neonatal pneumothorax (NP) are vital, as it may help avoid complications and reduce morbidity and mortality.2–4 Saudi Arabia does not have a nationalized registry for pneumothorax to strategize better health promotion towards the predominant risk factors and causative agents. Currently, there are limited studies on the epidemiology of NP from developing countries including the Middle East.5,6 Thus, we conducted this study to identify the incidence, clinical characteristics, predisposing factors, morbidity, and mortality among hospitalized neonates with pneumothorax. From this preliminary data, we aim to improve the standard of care and introduce clinical process improvement methods for neonates admitted to neonatal intensive care units (NICU) with pneumothorax.
Methods
We reviewed records of neonates with pneumothorax admitted to the NICU of King Fahad Medical City, Saudi Arabia, between 2011 and 2014. All newborns hospitalized in the NICU with chest X-ray confirmed pneumothorax were included in this study.
Patients were evaluated for the following: (1) baseline characteristics (gestational age, gender, maternal age, birth weight, Apgar score, and pneumothorax type), (2) predisposing factors of NP (steroids use, bag mask ventilation, continuous positive airway pressure (CPAP), mechanical ventilation, hypoplastic lung disease, and MAS), (3) accompanying disorders (premature rupture of membrane (PROM), intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA), retinopathy of prematurity (ROP), pulmonary hemorrhage, and necrotizing enterocolitis (NEC)), (4) treatment of NP (intervention, ventilation mode, oxygen duration, and length of hospital stay), and (5) outcomes (survived, died).
The subjects were categorized as term (≥ 37 weeks) or preterm (< 37 weeks) neonates according to their gestational age. Pneumothorax was divided into spontaneous pneumothorax (an intrapleural air collection in the absence of intubation, positive ventilation, or underlying pulmonary pathology) and secondary pneumothorax (with underlying lung pathology).7,8 Some subjects had more than one accompanying disorder or predisposing factor and these were recorded. Study approval was obtained from the hospital institutional review board.
Data was described as average (mean ± standard deviation (SD), median) and percentages. All the inferences were drawn at 95% confidence interval (CI). Chi-square and odds ratio (OR) were applied for non-metric data. Binary logistic regression analysis was done for survival analysis. A backward elimination approach in a multiple logistic-regression model was performed. In this model, the significant factors from the univariate analysis were removed one at a time, starting with the factor that had the largest p-value, until all remaining factors had a two-sided p-value < 0.050. OR and p-values were reported for each factor alone and for the factors found to be significant from the backward elimination. Data analysis was performed using SPSS Statistics (SPSS Statistics Inc., Chicago, USA) version 18, JavaStat, and MS Excel software.
Results
The most common predisposing factors of NP was bag mask ventilation in 24 (36.4%) neonates, followed by hypoplastic lung disease in 17 (25.7%) newborns, and mechanical ventilation in 14 (21.2%) neonates. Only six (9.1%) neonates had MAS as a predisposing factor [Figure 1]. The most common accompanying disorder was PROM in 77 (89.5%) neonates [Figure 2].
Figure 3 shows the modalities of treatment for NP. Forty-eight (55.8%) neonates were managed with chest drain while 34 (39.5%) did not need any management. The mean length of hospital stay was 26.5±4.4 days. The most utilized mode of ventilation was nasal cannula and mechanical ventilation in 31 (36.0%) and 30 (34.9%) neonates, respectively.
Table 1: Characteristics of neonates admitted with pneumothorax (n = 86).
|
Gender |
|
|
Male |
45 (52.3) |
|
Female |
41 (47.7) |
|
Birth weight, mean ± SD, g |
2 029.0 ± 1 063.5 |
|
< 2 500 |
48 (55.8) |
|
≥ 2 500 |
38 (44.2) |
|
Gestational age, mean ± SD, weeks |
33.1 ± 6.3 |
|
< 37 |
43 (50.0) |
|
≥ 37 |
43 (50.0) |
|
Maternal age, mean ± SD, years |
31.2±5.4 |
|
One minute Apgar score |
|
|
7–10 |
43 (50.0) |
|
4–6 |
29 (33.7) |
|
0–3 |
14 (16.3) |
|
Five minute Apgar score |
|
|
7–10 |
69 (80.2) |
|
4–6 |
14 (16.3) |
|
0–3 |
3 (3.5) |
|
Pneumothorax type |
|
|
Spontaneous |
20 (23.3) |
SD: standeard deviation.
Table 2: Survival analysis across studied factors among neonates with neonatal pneumothorax.
|
Gender |
|
Male |
10 (22.2) |
35 (77.8) |
0.5 [0.2–1.3] |
|
Female |
15 (36.6) |
26 (63.4) |
|
Birth weight, g |
|
< 2 500 |
22 (45.8) |
26 (54.2) |
9.9 [2.7–36.5]* |
|
≥ 2 500 |
3 (7.9) |
35 (92.1) |
|
Gestation age, weeks |
|
Preterm |
19 (44.2) |
24 (55.8) |
4.9 [1.7–14.0]* |
|
Term |
6 (14.0) |
37 (86.0) |
|
One minute Apgar score |
|
< 7 |
22 (51.2) |
21 (48.8) |
14.0 [3.7–52.1]* |
|
≥ 7 |
3 (7.0) |
40 (93.0) |
|
Five minute Apgar score |
|
< 7 |
11 (64.7) |
6 (35.3) |
7.2 [2.3–22.8]* |
|
≥ 7 |
14 (20.3) |
55 (79.7) |
|
Maternal age, years |
|
≤ 30 |
12 (28.6) |
30 (71.4) |
0.95 [0.4–2.4] |
|
> 30 |
13 (29.5) |
31 (70.5) |
|
Pneumothorax type |
|
Spontaneous |
0 (0.0) |
20 (100.0) |
0.04 [0.0-0.4]* |
|
Secondary |
25 (37.9) |
41 (62.1) |
|
PROM |
22 (28.6) |
55 (71.4) |
1.25 [5.0–0.3] |
|
MAS |
0 (0.0) |
6 (100.0) |
5.5 [0.5–57.1] |
|
PDA |
3 (23.1) |
10 (76.9) |
1.4 [0.4–5.7) |
|
NEC |
0 (0.0) |
2 (100.0) |
1.7 [0.1–19.9] |
|
IVH |
8 (42.1) |
11 (57.9) |
10.0 [0.7–6.3] |
|
ROP |
1 (11.1) |
8 (88.9) |
3.6 [0.4–30.6] |
|
Pulmonary hemorrhage |
5 (71.4) |
2 (28.6) |
5.0 [1.3–50.0]* |
*Significant p-value. OR: odda ratio; CI: confidence interval; PROM: premature rupture of membrane; MAS: meconium aspiration syndrome; PDA: patent ductus arteriosus; NEC: necrotising enterocolitis; IVH: intraventricular hemorrhage; ROP: retinopathy of prematurity.
Table 3: Binary logistic regression analysis of all significant variables.
|
Gestation age < 37 weeks |
2.0 [0.0–5.9] |
0.585 |
|
Birth weight < 2 500 g |
12.5 [1.15–100.0] |
0.038* |
|
One minute Apgar Score < 7 |
9.1 [1.7–50.0] |
0.010* |
|
Five minute Apgar Score < 7 |
2.5 [0.6–10.0] |
0.202 |
|
Pulmonary hemorrhage |
13.7 [1.7–114.4] |
0.015* |
*Significant p-value. OR: odda ratio; CI: confidence interval; IVH: intraventricular hemorrhage.
During the study period, 2 204 neonates were admitted to the NICU. NP was diagnosed in 86 patients (43 term, and 43 preterm), giving an incidence of 3.9%. Twenty-five (29.1%) neonates admitted with pneumothorax died during the study period. Out of the total neonates studied, 45 (52.3%) were male. The mean gestational ages and birth weights were 33.1±6.3 weeks and 2 029.0±1 063.5 g, respectively. In 66 (76.7%) neonates, the underlying cause of NP could be identified (secondary), whereas in 20 (23.3%) no reason for NP could be found (spontaneous) [Table 1].
When considered individually, a significant relationship existed between mortality and the birth weight, gestation age, Apgar scores at one and five minutes, type of pneumothorax, IVH, pulmonary hemorrhage, and steroid use [Table 2].
On binary logistic regression analysis, newborns with pulmonary hemorrhage had a 13.7-fold increased risk of dying from pneumothorax compared with newborns without pulmonary hemorrhage (p = 0.015). Moreover, birth weight < 2 500 g (p = 0.038) and low Apgar score (< 7) (p = 0.010) at one minute were independently associated with mortality [Table 3].
Discussion
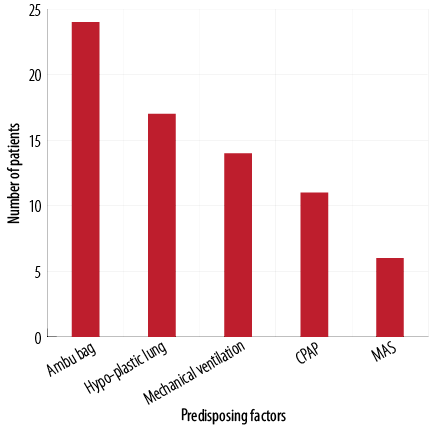
CPAP: continuous positive airway pressure; MAS: meconium aspiration syndrome.
Figure 1: Number of neonates with predisposing factors of neonatal pneumothorax (n = 66).
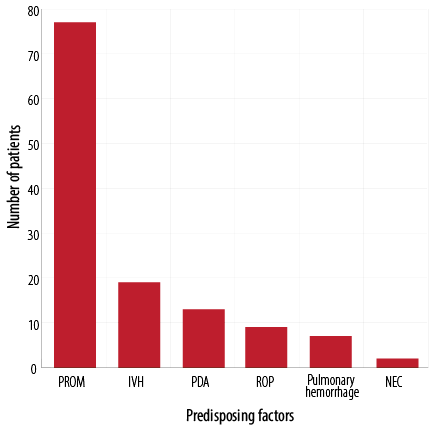
PROM: premature rupture of membrane, IVH: intraventricular hemorrhage; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity; NEC: necrotising enterocolitis.
Figure 2: Accompanying disorders in neonates with pneumothorax (n = 86).
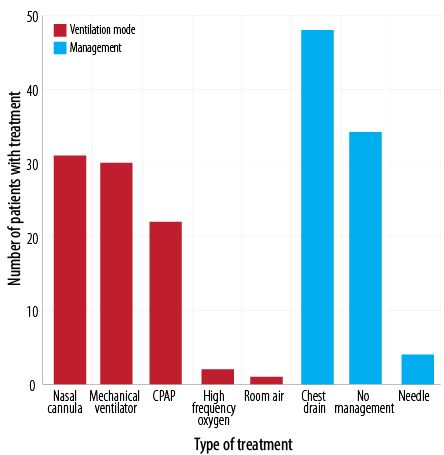
CPAP: continuous positive airway pressure.
Figure 3: Treatment and management of neonatal pneumothorax.
The first inspiratory effort in the infant produces transpulmonary pressure higher than 100 cm of the water column and opens the lungs that were collapsed in utero. After the first few breaths, this pressure is normalized, and the lungs start to function. If this transpulmonary pressure remains high for a long time, it leads to pneumothorax. This type of pneumothorax is recognized as spontaneous pneumothorax (primary). Secondary pneumothorax occurs in neonates with an underlying pathology like respiratory distress syndrome, MAS, pulmonary hypoplasia, as well as in resuscitated neonates.7,8 Pneumothorax has substantial mortality and morbidity effects on NICU patients. The incidence of NP in our NICU was 3.9%. Approximately, 29.1% of neonates in our study died. The reported incidence of NP in other studies was 0.5%–10%,9–11 and the mortality rate varied from 20% to 38%.12–16
Factors associated with pneumothorax are male sex, lower birth weight, prematurity, low Apgar score, and previous lung disease in the immediate perinatal period.7,17–20 Our study of demographic characteristics revealed that pneumothorax was more common among low birth weight neonates (< 2 500 g), neonates with secondary pneumothorax, and boys. A study conducted by O’Keefe et al,21 showed a higher ratio of boys to girls (1.66:1). Ngerncham et al,5 also found male sex to be one of the risk factors of pneumothorax during the first 24 hours of life. However, the greater incidence of pneumothorax in boys remains unexplained.
Predisposing factors of NP were mechanical ventilation, MAS, resuscitation, bag mask ventilation, and CPAP.22,23 In this study, underlying causes of NP were identified in 76.7% of neonates while NP was idiopathic in 23.3% of neonates. Bag mask ventilation was documented as the major cause of NP. The use of ventilator support during the neonatal period is necessary due to the frequent occurrence of a respiratory failure. Around 8.7% to 14% of ventilated neonates experienced at least one episode of NP. In our study, 14 (21.2%) neonates had mechanical ventilation as a predisposing factor of NP. There is an increased need for clinicians to improve mechanical ventilation strategies to reduce complications and improve patient outcomes.
The most common accompanying disorders in our study were PROM, IVH, and PDA. Similarly, another study also reported PROM and IVH as the most accompanying disorders.13
Asymptomatic pneumothorax does not necessitate specific treatment for infants without underlying pulmonary disease. Needle aspiration, chest tube insertion, and ventilator support should be considered if the patient presents with respiratory distress and poor blood gas changes.20 In our study, 34 (39.5%) neonates did not need any management, whereas chest drain/tube placement was done in 48 (55.8%) neonates, and 4 (4.7%) neonates were managed with needle aspiration.
The mortality rate was significantly higher in neonates with a low birth weight < 2 500 g, one minute Apgar score < 7, and pulmonary hemorrhage.
The Apgar score at five minutes was documented as a vital parameter in assessing the neonates’ well-being. Consistent with our results, a study conducted by Navaei et al,16 showed that there was no significant difference between mortality rate and Apgar score at five minutes. The mortality rate in neonates with gestation age < 37 weeks and IVH were not significantly associated with NP.
Because pneumothorax can cause sudden cardiopulmonary deterioration, early diagnosis and appropriate treatment can be crucial. Regardless of all the efforts in making an early diagnosis and treatment of the NP and the underlying diseases, the mortality rate is still high.
An important strength of the present study was that these preliminary data add to the understanding of the epidemiology of NP in Saudi Arabia. Our results will serve as the basis for future research and contribute to developing and optimizing strategies to combat these problems. This includes educating healthcare providers in the NICU about the factors that affect the morbidity and mortality of neonates with pneumothorax to improve the standards of NICU. The only study limitation was that our data were collected from a single hospital.
Conclusion
This study highlights the utility of conducting more extensive studies at a multicenter level in order to gauge the extent of NP problems among hospitalized neonates and common predisposing factors of NP. The mortality rate was shown to increase with low birth weight, one minute Apgar score < 7, and pulmonary hemorrhage. Further nationwide studies are needed to fully assess the magnitude of NP and provide a base for the development of general guidelines, and preventive programs for health education of healthcare providers working
in the NICU.
Disclosure
The authors declared no conflicts of interests. No funding was received for this study.
references
- 1. Seguier-Lipszyc E, Elizur A, Klin B, Vaiman M, Lotan G. Management of primary spontaneous pneumothorax in children. Clin Pediatr (Phila) 2011 Sep;50(9):797-802.
- 2. Litmanovitz I, Carlo WA. Expectant management of pneumothorax in ventilated neonates. Pediatrics 2008 Nov;122(5):e975-e979.
- 3. Hill A, Perlman JM, Volpe JJ. Relationship of pneumothorax to occurrence of intraventricular hemorrhage in the premature newborn. Pediatrics 1982 Feb;69(2):144-149.
- 4. Ogino M. Pulmonary air leak, Manual of neonatal care. 5th ed. Lippincott: Williams and Wilkins; 2004. 371-377.
- 5. Ngerncham S, Kittiratsatcha P, Pacharn P. Risk factors of pneumothorax during the first 24 hours of life. J Med Assoc Thai 2005 Nov;88(Suppl 8):S135-S141.
- 6. Boo NY, Cheah IG; Malaysian National Neonatal Registry. Risk factors associated with pneumothorax in Malaysian neonatal intensive care units. J Paediatr Child Health 2011 Apr;47(4):183-190.
- 7. Park SW, Yun BH, Kim KA, Ko SY, Lee YK, Shin SM. A clinical study about symptomatic spontaneous pneumothorax. Korean J Perinatol 2006;17:304-309.
- 8. Al Tawil K, Abu-Ekteish FM, Tamimi O, Al Hathal MM, Al Hathlol K, Abu Laimun B. Symptomatic spontaneous pneumothorax in term newborn infants. Pediatr Pulmonol 2004 May;37(5):443-446.
- 9. Chernick V, Avery ME. Spontaneous Alveolar Rupture at Birth. Pediatrics 1963 Nov;32:816-824.
- 10. Boedjang RF. Pneumothorax in the newborn with respiratory difficulties. Paediatr Indones 1990 Jul-Aug;30(7-8):198-203.
- 11. Wyatt TH. Pneumothorax in the neonate. J Obstet Gynecol Neonatal Nurs 1995 Mar-Apr;24(3):211-216.
- 12. Hansen T, Corbet A. Air block syndrome. In: Taeusch HW, Ballard RA eds. Avery’s Disease in the Newborn. 7th ed. Philadelphia: WB Saunders; 1998. p. 631-633.
- 13. Esme H, Doğru O, Eren S, Korkmaz M, Solak O. The factors affecting persistent pneumothorax and mortality in neonatal pneumothorax. Turk J Pediatr 2008 May-Jun;50(3):242-246.
- 14. Ainsworth AP, Ruager AR, Holtved E. [Neonatal pneumothorax]. Ugeskr Laeger 2000 Dec;162(49):6679-6682.
- 15. Ilçe Z, Gündogdu G, Kara C, Ilikkan B, Celayir S. Which patients are at risk? Evaluation of the morbility and mortality in newborn pneumothorax. Indian Pediatr 2003 Apr;40(4):325-328.
- 16. Navaei F, Aliabadi B, Moghtaderi M, Kelishadi R. Predisposing factors, incidence and mortality of pneumothorax in a neonatal intensive care unit in Isfahan, Iran. Zhongguo Dang Dai Er Ke Za Zhi 2010 Jun;12(6):417-420.
- 17. Trevisanuto D, Doglioni N, Ferrarese P, Vedovato S, Cosmi E, Zanardo V. Neonatal pneumothorax: comparison between neonatal transfers and inborn infants. J Perinat Med 2005;33(5):449-454.
- 18. Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al; NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007 Feb;196(2):147.e1-147.e8.
- 19. Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB, et al. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ 2004 Oct;329(7473):1004.
- 20. Zenciroğlu A, Aydemir C, Baş AY, Demirel N. [Evaluation of predisposing and prognostic factors in neonatal pneumothorax cases]. Tuberk Toraks 2006;54(2):152-156.
- 21. O’Keeffe FN, Swischuk LE, Stansberry SD. Mediastinal pseudomass caused by compression of the thymus in neonates with anterior pneumothorax. AJR Am J Roentgenol 1991 Jan;156(1):145-148.
- 22. Bhatia J, Mathew OP. Resolution of pneumothorax in neonates. Crit Care Med 1985 May;13(5):417-419.
- 23. Engdahl MS, Gershan WM. Familial spontaneous pneumothorax in neonates. Pediatr Pulmonol 1998 Jun;25(6):398-400.