Globally, the irrational use of antibiotics constitutes a fundamental factor related to the emergence of antibiotic resistance, which is why the implementation of antimicrobial stewardship programs in healthcare facilities is highly recommended.1,2 The expected benefits include improved patients outcomes, prevention of adverse events, and the emergence of antimicrobial resistance. In addition, the rational use of antibiotics has a significant impact on healthcare efficiency.3 Monitoring antibiotic consumption is a recommended component of antimicrobial stewardship programs, providing information about the pattern and trends of consumption at the facility level and for interfacility comparison. In addition, data about consumption would provide evidence of improper use and the need to conduct additional studies or interventions. According to the recently published guidelines, days of therapy (DOT) and defined daily dose (DDD) are standardized methods for monitoring consumption.2
In a non-teaching Lebanese hospital amoxicillin-clavulanate, ceftriaxone, amoxicillin, and cefuroxime were the most common antibiotics used.4 Another study identified a significant frequency of inappropriate antibiotic use in a teaching hospital.5
Limited information has been published about antibiotic use in Qatar. Available studies have described the prevalence of antimicrobial use (43%) in oncology patients and the inappropriateness of antimicrobial prophylaxis (53.3%) in a general hospital.6,7 Similarly, there is evidence about the high prevalence of multidrug-resistant organisms in Qatar and neighboring countries, mainly in patients with healthcare-associated infections.8–11 There is also evidence of the elevated incidence of carriers of extended-spectrum-β-lactamase organisms.12
The aim of our study was to evaluate the trend of antibiotic consumption in patients admitted to a community hospital with an antimicrobial stewardship program in Qatar over four years from 2012 to 2015.
Methods
An observational study was carried out over a 4-year period (from 2012 to 2015) in a 75-bed facility that serves a population of western Qatar with outpatient and emergency services, inpatient wards for clinical and surgical patients (adults and pediatrics), a gynecology/obstetric ward, and critical care units (adults and neonatology).
The use of antibiotics is guided by a corporate policy (Policy CL 9197 Antimicrobial Prescribing) for therapeutic use and surgical prophylaxis, which includes additional measures such as antibiotic stop orders, switching from intravenous (IV) to oral antibiotics, and antibiotic restriction.
The local antimicrobial stewardship program includes the following components:
- Audit of the quality of prescriptions on an ongoing basis with a special emphasis on antibiotic prophylaxis in surgery, community-acquired pneumonia (CAP), and restricted drugs. Additional targeted audits conducted based on results of antimicrobial consumption data or other evidence on inadequate prescriptions.
- Monitoring of antimicrobial consumption by a pharmacist using pharmacy records and presented as DDD by patient days and expressed as 100 bed-days (DBD), as per the Anatomical Therapeutic Chemical (ATC) classification and DDD methodology.8 Consumption was presented according to the antibiotic family (cephalosporins, penicillins, carbapenems, aminoglycosides, and fluoroquinolones) and the most frequent IV and oral antibiotics.
- Feedback about the quality of prescriptions and antimicrobial consumption to medical staff and leaders. A monthly report was generated and distributed by email.
- Data presented in Quality and Patient Safety committees, including the Infection Prevention and Control Committee and Pharmacotherapeutic Committee.
- Training staff using a diverse range of activities including lectures, case presentations, and analysis of non-compliance.
Descriptive statistical analysis was performed using JMP Statistical Discovery Software version 10.0 (SAS Institute Inc), and the t-test was applied to evaluate the changes in consumption in 2015 compared to 2014. The 95% confidence interval (CI) was calculated for antimicrobials consumption. A p-value ≤ 0.050 was considered significant.
Results
A sustained increased in admissions was observed during the study period with 281 admissions in 2012 and 1278, 3052, and 3741 in 2013, 2014, and 2015, respectively. In 2012, the total consumption of antibiotics was 59.7 DBD (95% CI 53.9–65.4), decreasing to 44.4 (95% CI 41.7–47.2), 46.7 (95% CI 44.9–48.5), and 38.3 (95% CI 36.7–39.9) in 2013, 2014, and 2015, respectively. A significant decrease in consumption was observed in 2015 compared to 2014 (18.0%) and with 2012 (35.8%).
The use of cephalosporins reduced from 17.3 DBD (95% CI 15.3–19.4) in 2013 to 10.7 DBD (CI 95% 9.7–11.7) in 2015 while the consumption of penicillin increased during the beginning of 2014 with a slight decrease in 2015. The use of carbapenems during 2014–2015 was lower than the previous years and vice-versa for aminoglycosides. Fluoroquinolones had a sustained increase with 40.5% more consumption in 2015 compared to 2014 [Figure 1 and 2].
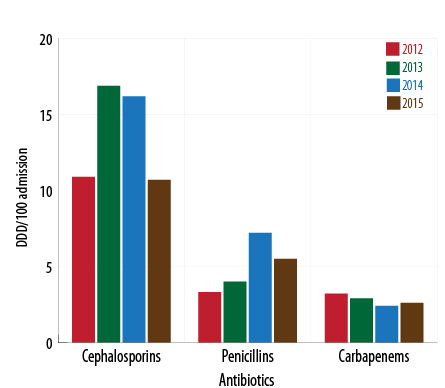
Figure 1: Consumption of β-lactam antibiotics and year (2012-2015)(DDD/100 patient days) .
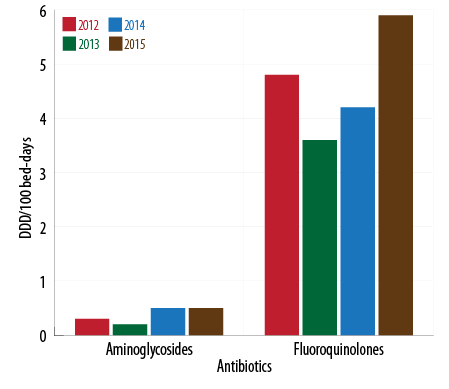
Figure 2: . Fluoroquinolones and aminoglycosides consumption by year (2012-2015).
Annual consumption of the ten most frequently used IV and oral antimicrobials, and the proportional change in 2015 compared with 2014 is shown in Table 1. Ceftriaxone (33.0%) and cefuroxime (15.0%) had significant reductions (p < 0.001) in consumption and were mainly prescribed for CAP in adults and antibiotic prophylaxis, respectively. Metronidazole was mainly prescribed for antibiotic prophylaxis, treatment after contaminated or dirty surgical procedures. We observed reduced consumption of IV (21.1%) and oral forms (25.0%) of the drug.
Table 1: Antibiotic consumption between 2012 and 2015 (DDD/100 bed-days).
|
IV |
|
|
|
|
|
|
ceftriaxone |
19.2 (14.6–23.8) |
11.8 (10.0–13.6) |
10.0 (11.1–8.9) |
6.7 (5.9–7.5) |
-33.0* |
|
metronidazole |
4.9 (2.4–7.4) |
3.4 (2.4–4.4) |
5.7 (4.9–5.6) |
4.5 (3.8–5.2) |
-21.1* |
|
cefuroxime |
7.0 (4.0–9.9) |
3.3 (2.3–4.3) |
4 (3.3–4.7) |
3.4 (2.8–3.0) |
-15.0* |
|
meropenem |
3.2 (1.1–5.3) |
2.6 (1.7–3.5) |
2.4 (1.9–2.9) |
2.6 (2.1–3.1) |
+8.3 |
|
piperacillin/tazobactam |
0.2 (0–0.32) |
0.9 (0.4–1.4) |
2.2 (1.7–2.7) |
2.2 (1.7–2.7) |
0.0 |
|
azithromycin |
0 |
1.0 (0.5–1.6) |
0.9 (0.6–1.2) |
2.0 (1.6–2.5) |
+122.2* |
|
moxifloxacin |
0 |
0.7 (0.2–1.2) |
1.0 (0.7–1.4) |
1.2 (0.9–1.6) |
+20.0** |
|
trimethoprim/sulfamethoxazole |
0 |
0 |
0.3 (0.1–0.5) |
1.0 (0.7–1.3) |
+ 233.3* |
|
ampicillin |
0 |
0.9 (0.4–1.4) |
1.0 (0.7–1.4) |
0.7 (0.4–1.0) |
- 30.0** |
|
ciprofloxacin |
4.1 (1.8;6.4) |
1.4 (0.8–2.0) |
1.0 (0.7–1.4) |
0.6 (0.4–0.9) |
- 40.0* |
|
Oral |
|
|
|
|
|
|
moxifloxacin |
0 |
0.5 (0.1–0.9) |
1.1 (80.7–1.5) |
2.8 (2.3–3.3) |
+ 154.5* |
|
azithromycin |
9.7 (6.2–13.2) |
8.1 (6.6–9.6) |
5.2 (4.4–6.0) |
2.4 (1.9–2.9) |
- 53.8* |
|
amoxicillin/clavulanic acid |
0.9 (0–0.20) |
1.9 (1.2–2.7) |
3.9 (3.2–4.6) |
2.4 (1.9–2.9) |
- 38.5* |
|
doxycycline |
1.3 (0–2.6) |
0.3 (0–0.6) |
1.0 (0.7–1.4) |
1.1 (0.8–1.4) |
+ 10.0 |
|
ciprofloxacin |
0.8 (0–1.8) |
0.9 (0.4–1.4) |
0.9 (0.6–1.2) |
0.8 (0.5–1.1) |
- 11.1 |
|
trimethoprim/sulfamethoxazole |
0 |
0 |
0.5 (0.3–0.8) |
0.6 (0.4–0.9) |
+ 20.0 |
|
levofloxacin |
0 |
0 |
0.2 (0.1–0.4) |
0.5 (0.3–0.7) |
+ 150.0* |
|
metronidazole |
0.2 (0–0.7) |
1.1 (0.5–1.7) |
0.4 (0.2–0.6) |
0.3 (0.1–0.5) |
- 25.0 |
|
amoxicillin |
2.0 (0.4–3.6) |
0.2 (0–0.4) |
0.1 (0–0.2) |
0.2 (0.06–0.3) |
+ 100.0* |
#Data of the ten most frequently used IV or oral antibiotics were included. ##Data presented as DDD/100 bed-days (95% CI).
*p < 0.001; **p < 0.050.
Azithromycin and moxifloxacin are prescribed, according to local policies, for the management of CAP in adults and uncompensated chronic obstructive pulmonary disease. We observed an increase in the use of IV (122.2%) and oral (53.8%) azithromycin while the use of oral moxifloxacin (154.5%) was higher than the increase in parenteral forms (20.0%). Ciprofloxacin use was less in IV and oral forms while oral levofloxacin increased 150.0%.
IV (233.3%) and oral (20.0%) trimethoprim/sulfamethoxazole were used more in 2015 compared to 2014. Similarly, oral amoxicillin consumption (100.0%) increased significantly in 2015.
Discussion
Our data showed that antibiotic consumption reduced from 2012 to 2015, mainly due to the reduction in cephalosporins consumption. The high use of antibiotics during 2012 compared with the later years could be related to the limited measures implemented to control antimicrobial prescriptions.
The favorable trends in consumption of cephalosporin may be due to improvements in the quality of antibiotic prophylaxis for surgical procedures and the timely switch from IV to oral therapy, mainly in CAP. According to unpublished data, a reduction of cefuroxime use in appendectomies of 27% DDD/100-procedures was observed in 2015 compared to 2013. The association of ceftriaxone plus azithromycin constituted the first options of empiric therapy for CAP and is the most frequent reason for prescribing this cephalosporin. The reduction in its consumption is mainly related to a switch to oral antibiotics in these patients.
The most frequently used penicillins were IV piperacillin-tazobactam (Pip/Taz) and oral amoxicillin-clavulanate, which were prescribed in patients with community and healthcare-associated infections. Pip/Taz is, according to local policy, the drug of choice for severe CAP, a frequent cause of admission in adult critical care units. Similarly,
Al-Yamani13 described the frequent use of Pip/Taz in patients admitted to acute care wards. Monitoring antimicrobial consumption has been considered an essential component of antimicrobial stewardship programs, in addition to auditing the quality of prescriptions for therapeutic or prophylactic use in the different settings during patient care, including the emergency department, inpatient, or outpatient clinics.14,15
Fluoroquinolones were the only antimicrobial class that increased in use during the period, an issue that should be considered due to the high frequency of tuberculosis in the expatriate population living in Qatar.16 The greatest increase in consumption was for moxifloxacin, mainly related to its use for the switch from IV to oral treatment in CAP. Physician preferences for prescribing moxifloxacin could be associated with its antimicrobial spectrum and dosage. Although many published studies recommend moxifloxacin for treatment of CAP, several studies reported the effect of fluoroquinolones treatment in the delay of tuberculosis diagnosis.17,18 Consequently, we recommend in our setting the prescription of moxifloxacin after the diagnosis of tuberculosis has been ruled out in non-severe cases and the collection of laboratory samples for acid-fast bacilli identification before the initiation of the treatment.
The reduction in antimicrobial consumption in relation with the achievements of clinical outcomes and prevention of other adverse effects, including mortality and the incidence of Clostridium difficile infections, have a definitive impact on the optimization of resources.19,20 Hospital-wide or focused selected antimicrobials stewardship programs have demonstrated improvement in the quality of prescriptions and the frequency of antimicrobial use in many settings.21–23 The antimicrobial stewardship program used well-known components as monitoring the quality of prescription and feedback, antibiotic restriction, and others already mention. Monitoring of consumption is considered an important part of our local program because it allows us to identify the pattern and trends of consumption. Primarily, this measure does not identify deficiencies in the quality of prescriptions, but it provides information to conduct additional studies on this issue. Although evaluating the outcomes of the antimicrobial stewardship program was not the primary purpose of this paper, during the study period only one patient had healthcare-associated C. difficile infection, and five patients had methicillin-resistant Staphylococcus Aureus infections (MSRA).
As a single center experience, our data can be used to evaluate consumption and trends at the facility level, but cannot be compared to other facilities. The differences in the patient populations, the procedures and others factors that define the complexity of the facilities constituted critical factors to analyze the consumption of antimicrobials. In addition, we have no patient level data or information about the consumption for a specific disease or procedure. This will limit the possibility to identify focused strategies for improvement. Nevertheless, the ongoing monitoring of antibiotic prophylaxis in surgery and targeted audits performed has contributed to minimizing this limitation.
Conclusion
This study shows a decrease in antibiotic utilization in patients admitted to a community hospital with an antimicrobial stewardship program. There was an increase in fluoroquinolones consumption, which is a concern and requires focused strategies.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to thank Mr. Carlos L Crespo Palacios for his assistance in reviewing this paper.
references
- 1. Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012 Apr;33(4):322-327.
- 2. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016 Apr 13;ciw118.
- 3. Guanche Garcell H, Pisonero Socias JJ, Enseñat Sánchez R, Fiterre Lancis I, Mir Narbona I, García Arzola B, et al. Impacto de un programa de control de la calidad de la prescripción de antibióticos en un hospital de La Habana, Cuba. Rev Panam Salud Publica 2011 Dec;30(6):598-602.
- 4. Iskandar K, Hanna PA. Salameh P, Raad EB. Antibiotic consumption in non-teaching Lebanese hospitals: A cross-sectional study. J Infect Public Health 2016 Jan 21.[Epub ahead of print].
- 5. Bozkurt F, Kaya S, Tekin R, Gulsun S, Deveci O, Dayan S, et al. Analysis of antimicrobial consumption and cost in a teaching hospital. J Infect Public Health 2014 Mar-Apr;7(2):161-169.
- 6. Hammuda A, Hayder S, Elazzazy S, Black E. Point prevalence survey of antimicrobial utilization in oncology patients. J Infect Dev Ctries 2013 Dec;7(12):990-993.
- 7. Abdel-Aziz A, El-Menyar A, Al-Thani H, Zarour A, Parchani A, Asim M, at al. Adherence of surgeons to antimicrobial prophylaxis guidelines in a tertiary general hospital in a rapidly developing country. Adv Pharmacol Sci. 2013;2013:842593.
- 8. Aly M, Balkhy HH. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob Resist Infect Control 2012;1(1):26.
- 9. Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev 2013 Jul;26(3):361-380.
- 10. Guanche Garcell H. Antibiotic prophylaxis in surgical procedures and the threats of fecal carriage of the extended spectrum? lactamase producer organisms. Qatar Foundation Annual Research Conference 2016. Conference Proceedings: HBPP1550; Qatar: Hamad bin Khalifa University Press; 2016.
- 11. Guanche Garcell H. Fernandez Hernandez TM Abu Baker Abdo E, Villanueva Arias A. Evaluation of the timeliness and completeness of communicable disease reporting: Surveillance in The Cuban Hospital, Qatar. Qatar Med J 2014 Jun 6;2014(1):50-56.
- 12. World Health Organization Collaborating Center for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2010. WHO: Oslo; 2009. [cited 2016 February 8]. Available from: http://www.whocc.no/atcddd/.
- 13. Al-Yamani A, Khamis F, Al-Zakwani I, Al-Noomani H, Al-Noomani J, Al-Abri S. Patterns of Antimicrobial Prescribing in a Tertiary Care Hospital in Oman. Oman Med J 2016 Jan;31(1):35-39.
- 14. Al-Niemat SI, Aljbouri TM, Goussous LS, Efaishat RA, Salah RK. Antibiotic Prescribing Patterns in Outpatient Emergency Clinics at Queen Rania Al Abdullah II Children’s Hospital, Jordan, 2013. Oman Med J 2014 Jul;29(4):250-254.
- 15. Gunn B, Ali S, Abdo-Rabbo A, Suleiman B. An Investigation into Perioperative Antibiotic Use during Lower Segment Caesarean Sections (LSCS) in Four Hospitals in Oman. Oman Med J 2009 Jul;24(3):179-183.
- 16. Report AH. Department of Epidemiology & Medical Statistics. Hamad Medical Corporation, 2013.
- 17. Kuzman I, Bezlepko A, Kondova Topuzovska I, Rókusz L, Iudina L, Marschall H-P, et al. Efficacy and safety of moxifloxacin in community acquired pneumonia: a prospective, multicenter, observational study (CAPRIVI). BMC Pulm Med 2014;14:105.
- 18. Grossman RF, Hsueh PR, Gillespie SH, Blasi F. Community-acquired pneumonia and tuberculosis: differential diagnosis and the use of fluoroquinolones. Int J Infect Dis 2014 Jan;18:14-21.
- 19. Bao L, Peng R, Wang Y, Ma R, Ren X, Meng W, et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010-2014. PLoS One 2015;10(3):e0118868.
- 20. Wu CT, Chen CL, Lee HY, Chang CJ, Liu PY, Li CY, et al. Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guidedantimicrobial stewardship program in a local hospital. J Microbiol Immunol Infect 2015 Nov 19. [Epub ahead of print].
- 21. Borde JP, Kaier K, Steib-Bauert M, Vach W, Geibel-Zehender A, Busch H, et al. Feasibility and impact of an intensified antibiotic stewardship programme targeting cephalosporin and fluoroquinolone use in a tertiary care university medical center. BMC Infect Dis 2014;14:201.
- 22. Scholze K, Wenke M, Schierholz R, Groß U, Bader O, Zimmermann O, et al. The Reduction in Antibiotic Use in Hospitals. Dtsch Arztebl Int 2015 Oct;112(42):714-721.
- 23. Borde JP, Kern WV, Hug M, Steib-Bauert M, de With K, Busch HJ, et al. Implementation of an intensified antibiotic stewardship programme targeting third-generation cephalosporin and fluoroquinolone use in an emergency medicine department. Emerg Med J 2015 Jul;32(7):509-515.