|
INTRODUCTION
Peracetic acid (PAA) is widely used in the food industry for disinfecting buildings and equipment and for sterilizing plastic bottles; particularly those used for packaging fruit juices or sweetened drinks. It is also used for medical purposes, mainly for sterilizing the surface of instruments.1 It has been accepted worldwide in the food processing and beverage industries as being ideal for clean-in-place systems. It is also used as a sanitizer in the pharmaceutical and cosmetic industries.1-2 It is well identified that water disinfection such as sodium hypochlorite, chlorine dioxide and peracetic acid through chlorination cause the formation of a mixture of disinfection by-products, many of which are genotoxic and carcinogenic.3 The disinfection process is an effective barrier to many pathogens in drinking water. However, many studies have shown that the presence of mutagens in drinking water is due not only to different pollution sources but also to disinfection treatment.4-16 Peracetic acid is worth studying for its application in drinking water disinfection. Data from peracetic acid studies do not raise immediate concern as to mutagenicity,17-18 and carcinogenicity.19 Conti et al. evaluated the genotoxicity of two widely used drinking water disinfectants, 0.1 to 0.5 mg/l sodium hypochlorite (NaClO)-chlorine dioxide (ClO2), and Peracetic acid (PAA, CH3-CO-COOH5).20 The disinfecting of peracetic acid is based on oxidative reactions, but very little is known about the possible risk of the different concentrations of peracetic acid on human health.17 It has been reported that peracetic acid treatment may cause the production of free radicals and halogenated organic by-products.21 There are few investigations about the health effects of administration of peracetic acid in human contact material and disinfection of drinking water and foods. This paper assesses the possible damage, due to the different concentrations of peracetic acid in drinking water, on serum lipid peroxidation (expressed as malondialdehyde (MDA) and hepatic enzymes (Alanine transaminase (ALT) and Aspartate transaminase (AST)) that can be a useful tool for the evaluation of human risk assessment.
METHODS
Peracetic acid (PAA) was purchased from Behban Chemistry Company Gorgan, Iran. All other chemicals such as analytical grade were obtained from Merck (Germany).
The study was performed in 2008 on 48 albino male Wistar rats of 10–12 postnatal weeks (provided from the Iranian Pasteur Institute) in the Faculty of Medicine, Gorgan University of Medical Sciences. The rats were randomly divided into six equal case groups on the basis of the differences between the periods of exposure to PAA:
Treatment Group (Group I) received 0.2% PAA daily for 2 weeks.
Treatment Group (Group II) received 2% PAA daily for 2 weeks.
Treatment Group (Group III) received 20% PAA daily for 2 weeks.
Treatment Group (Group I) received 0.2% PAA daily for 4 weeks.
Treatment Group (Group II) received 2% PAA daily for 4 weeks.
Treatment Group (Group III) received 20% PAA daily for 4 weeks.
There were also six control groups that only received drinking water daily for 2 and 4 weeks. All animals had free access to solution as drinking water. Approval for this study was obtained from the Gorgan University of Medical Sciences, animal Care and Ethics Committee. It was found by means of a digital scale that the mean weight for each group was 198.83 g (I), 155.83 g (II), 186.0 g (III) and 202.50 g (control group).
The PAA purchased was 99% pure. By dissolving 0.2, 2 and 20 ml of this PAA in 100 ml drinking water, PAA solutions of each dosage of solution was prepared.
When the experiments had expired, each of the rats of the six experiments and two control groups were anesthetized with ether, 5 ml blood were taken from the heart (right ventricle) and collected in tubes for Lipid peroxidation and hepatic enzymes investigations.
Then serum lipid peroxidation (expressed as malondialdehyde) and hepatic enzymes (Alanine Transaminase and Aspartate Transaminase) were examined by Satoh Method,22 and Commercial laboratory kit, and by using specterophotometry technique (model JENWAY 6105 UV/VIS) in Biochemistry and Metabolic Disorder Research Center (Faculty of Medicine).
2.5 ml of trichloroacetic acid was added to 0.5 ml plasma and the tube was left to stand for 10 min at room temperature. After centrifugation at 3500 rev./min for 10 min, the supernatant was decanted and the precipitate was washed once with sulfuric acid. Then 2.5 ml sulfuric acid and 3 ml thiobarbituric acid (TBA) in sodium sulfate were added to this precipitate and the coupling of lipid peroxide with TBA was carried out by heating in a boiling water bath for 30 min. After cooling in cold water, the resulting chromogen was extracted with 4 ml of n-butyl alcohol by vigorous shaking. Separation of the organic phase was facilitated by centrifugation at 3000 rev./min for 10 min and its absorbance was determined at the wavelength of 530 nm.
Experimental results concerning this study were evaluated using SPSS v.11.5 (ANOVA test) and expressed as Mean± SD. Also, Post huc (Turkey test) was performed to determine the difference between the groups and p<0.05 was considered significant.
RESULTS
Findings of serum Malondialdehyde (MDA), Alanine Transaminase (ALT) and Aspartate Transaminase (AST) are reported in Tables 1 and 2, which contain mean ± SD of MDA, ALT and AST values for rats exposed to 0.2%, 2% and 20% Peracetic acid daily for 2 (Table 1) and 4 (Table 2) weeks and the control groups.
The mean value of serum MDA (4.57±0.28 nmol/ml) increased and serum ALT (26.50±3.10 IU/L) and AST (25.75±2.21 IU/L) decreased significantly in group III, in comparison with the control group (MDA: 2.58±0.63 nmol/ml, ALT: 37.70±4.12 IU/L and AST:35.75±4.34 IU/L), (p<0.05). There were also significant increases in MDA and decreases in ALT and AST in group III when compared with group II (MDA: 2.75±0.23 nmol/ml, ALT: 35.0±3.55 IU/L and AST: 34.50±3.31 IU/L) and group I (MDA: 2.62±0.38 nmol/ml, ALT: 36.75±1.50 IU/L and AST: 35.25±4.57 IU/L), (p<0.05). There was no significant difference between group I, II and the control group (Table 1; 2 weeks exposed to peracetic acid).
The mean of serum MDA (5.03±0.46 nmol/ml) increased and serum ALT (23.75±1.50 IU/L) and AST (27.25±1.50 IU/L) decreased significantly in group II when compared with the control group (MDA: 2.62±0.40 nmol/ml, ALT: 35.43±7.27 IU/L and AST:33.75±2.62 IU/L), (p<0.05). There was also a significant increase in MDA and decrease in ALT and AST in group II (MDA: 5.03±0.46 nmol/ml, ALT: 23.75±1.50 IU/L and AST: 27.25±1.50 IU/L) when compared with group I (MDA: 2.67±0.09 nmol/ml, ALT: 34.50±1.29 IU/L and AST: 32.50±2.08 IU/L), (p<0.05). There was no significant difference between group I and the control group (Table 2, 4 weeks exposed to peracetic acid). The treatment group III that received 20% PAA daily for 4 weeks died.
Table 1: Mean± SD of serum malondialdehyde (MDA), Alanine transaminase (ALT) and Aspartate transaminase (AST) in control and treatment groups (received 0.2, 2 and 20% peracetic acid daily for 2 weeks).
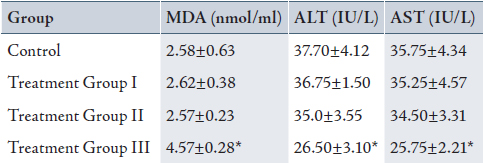
Values are means ± SD. Calculated from n=6 in each group.
* p<0.05 compared to control, Treatment Group I and II (ANOVA).
(I): Treatment Group received 0.2% PAA daily for 2 weeks.
(II) Treatment Group received 2 % PAA daily for 2 weeks.
(III) Treatment Group received 20% PAA daily for 2 weeks.
Table 2: Mean± SD of serum malondialdehyde (MDA), Alanine transaminase (ALT) and Aspartate transaminase (AST) in control and treatment groups (received 0.2, 2 and 20% peracetic acid daily for 4 weeks).
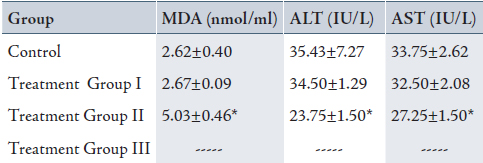
Values are means ± SD. Calculated from n=6 in each group.
* p<0.05 compared to control and Treatment Group I (ANOVA).
(I): Treatment Group received 0.2% PAA daily for 4 weeks.
(II) Treatment Group received 2% PAA daily for 4 weeks.
(III) Treatment Group received 20% PAA daily for 4 weeks (no sampling).
DISCUSSION
Drinking water quality is a health concern in countries at all levels of economic development and microbial hazards continue to be a primary worry. Therefore, disinfection is of unquestionable importance in the supply of safe drinking water. The disinfection process is an effective barrier to many pathogens, especially bacteria, and should be used for surface waters and ground water subject to faecal contamination. Residual disinfection is used to provide a partial safeguard against low level contamination and re-growth within the distribution system. However, the use of chemical disinfectants in water treatment usually results in the formation of by-products that can be toxic and/or mutagenic.23-31 Although drinking water has certainly a greater potential to impact human health, wastewater contamination may also pose health risks.
The aim of this study was to evaluate the effect of peracetic acid on lipid peroxidation and hepatic enzymes in Wistar rats. Findings of this study showed that lipid peroxidation increased and hepatic enzyme activities (ALT and AST) decreased in peracetic acid treatment groups when compared with control groups. The increased lipid peroxidation observed in this study is in agreement with the finding of Marabini et al. who found a statistically significant increase in reactive oxygen species in hepatocyte cells exposed for 2 hours to peracetic acid.3 The study by Grant et al. showed that the oxidative damage could be completely prevented when peracetic acid exposed to yeast by the highly active mitochondrial defends against reactive oxygen species in yeast.32 There is limited information about toxicity of PAA on lipid peroxidation and hepatic enzymes. However some investigators have studied the effects of PAA on various organs. Muller et al. investigated chronic Peracetic acid (wofasteril) administration on the rabbit oral mucosa, vaginal mucosa, and skin.19 In the mentioned study, mucosal tissues showed no inflammations after administration of Peracetic acid. But a study by Laub et al. indicated damage to Langerhans cell population after application of Peracetic acid.33 Also, the results of Wutzler et al. showed that higher concentrations of Peracetic acid lead to considerable changes of the epithelium of the urinary bladder in the form of partly focal partly diffuse hemorrhagic necrotizing urocystitides.34 Moreover, Peracetic acid at 3% caused dermatitis on guinea pig skin. Findings of Heinze et al. revealed Bronchopneumonia and liver granuloma in Mice and guinea pigs who were exposed to Peracetic acid aerosol (186 or 280 mg/ml) 30 min twice daily for 90 days also, increased incidence of lung tumors and decreased leukocyte counts were observed in mice.35 It is known that Peracetic acid through chlorination causes the formation of a mixture of disinfection by-products (DBPs), many of which are genotoxic and carcinogenic.3 Increased risk of liver, kidney and intestinal cancers in rodents, and intestinal tumors and low birth weight in humans has been associated with disinfection by-products.36 The application of chlorinated disinfectants such as Peracetic acid during drinking-water production has been shown to generate halogenated compounds, that induce genotoxic effect consumption of chlorinated drinking-water which has been correlated with increased risk for cancer induction in human populations.37 The primary mode of PAA action is oxidation. PAA disinfects by oxidizing of the outer cell membrane of cells. The mechanism of oxidation is the transfer of electrons, therefore the stronger the oxidizer, the faster electrons are transferred to cells and the faster the cells is inactivated or killed. Therefore PAA has a higher oxidation potential than chlorine sanitizers.
PAA also inactivates enzymes that are responsible for discoloration and degradation, such as peroxidase.38 According to US National Library of Medicines, Peracetic acid caused oxidative stress and subsequent disruption of their cell membrane, via the hydroxyl radical (OH).39 As diffusion of chemical is slower than the half-life of the radical, it will react with any oxidizable compound in its vicinity. It can damage virtually all types of macromolecules associated with a microorganism; carbohydrates, nucleic acids (mutations), lipids (lipid peroxidation) and amino acids (e.g. conversion of Phe to m-Tyr and o-Tyr). This ultimately leads to cell lysis and true microbial death.40 Peracetic acid’s mechanism of action is hypothesized to be the denaturation of proteins and enzymes and increased cell wall permeability by breaking sulfhydryl and disulfide bonds.40-41 These effects could result in increased lipid peroxidation and decreased enzyme activities. The decreased activities of hepatic enzymes maybe due to the increased level of lipid peroxidation and possible damage to hepatocyte cells when rats were exposed to high concentration of peracetic acid (20% and 2%) for 2 and 4 weeks respectively.
CONCLUSION
The results obtained in this study suggest that serum MDA, ALT and AST with 0.2% and 2% doses of peracetic acid for 2 weeks does not lead to the alteration of MDA and enzyme activities. These results point out that the enhancement of the MDA could provide an oxidative damage induced by disinfectant peracetic acid at 20% and 2% doses at 2 and 4 weeks respectively. Thus oral consumption of peracetic acid with concentration of 20% for 2 weeks and 2% for 4 weeks can cause increase and decrease lipid peroxidation and hepatic enzyme activities (ALT and AST) in animal model respectively.
ACKNOWLEDGEMENTS
The authors would like to thank the personnel at the Biochemistry and Metabolic Research Center of Golestan University of Medical Sciences for providing for their cooperation and assistance in the handling of experiments. There were no conflicts of interest and no funding was received on this work.
|