Proceedings of Second International Emergency Medicine
and Disaster Management Conference
17 – 19 March 2009. Muscat, Sultanate of Oman
Introduction
by
Ammar Al-Kashmiri, MD, FRCP(C), DABEM
Emergency
Medicine Physician
Chairman,
Scientific Committee
2nd
International Emergency Medicine and Disaster Management conference
Emergency Medicine has made the headlines!
This young specialty was recently celebrated between 17-19 March 2009, during
the Second International Emergency Medicine and Disaster Management conference
which was held in the Grand Hyatt Hotel in Muscat. This scientific assembly was
a huge success by all means. The conference ran over two and a half days and
covered a wide range of topics related to the specialty. The talks were
delivered by renowned experts in their corresponding subspecialties of
Emergency Medicine, who came from different parts of the globe including North
America, Europe, Australia and neighboring
Saudi Arabia. This versatile mix of expertise lead to an important and
interesting exchange of knowledge and was definitely an enriching factor. The
speakers were not only experts in their fields but also had great dynamicity in
delivering their presentations which kept the audience engaged at all times.
The first day began with a prestigious
opening ceremony attended by many high ranking officials from different
government sectors. During the ceremony a talk was delivered by Dr. Scott
Delaney, an Emergency Physician from Canada, who reflected on Emergency
Medicine as a specialty, how it started, where it is at present and where it is
heading. This constituted a great introduction to the conference and emphasized
that Emergency Medicine is a well established specialty with its own area of
knowledge and complex set of skills. The future of Emergency Medicine in Oman,
no different from other parts of the developed world, can only made to flourish
by the country investing in its own physicians. The day continued to cover the
main focus of the conference namely Disaster Management. This special focus was
felt to be essential in view of the recent cyclone Gonu
that hit the country in the summer of 2007 subjecting its medical system to
great challenges and putting it through the test.
Oman is also unfortunately suffering from
an epidemic of road traffic accidents causing devastating disability and death.
From this perspective the program included several sessions covering topics
related to Trauma and EMS.
Other areas covered included Cardiology,
Critical Care, Pediatric Emergency, Aviation
Medicine, Research and Medical Education.
The conference also had a local flavor. Several talks were delivered by local speakers who
shared their valuable experiences in Disaster, EMS and other fields. A full
session was dedicated to cover issues related to the practice of Emergency
nursing delivered by local experts in this field.
The conference was attended by about 600
delegates. The audience was a mix of physicians, residents, nurses and
paramedics.
Overall, there was excellent positive
feedback from speakers and delegates and the experience was enriching to
everyone:
“Thanks again for the wonderful hospitality
and excellent conference. I really enjoyed seeing your great country for the
first time and got good academic value from the other speakers, too...”
Dr. Peter Jones, Auckland, New Zealand
“We have not stopped talking about our trip
and the conference. The warm welcome from all, the excellent organisation and
the hard work of so many, not only contributed to making the conference a huge
success but also made the speakers feel valued and at home in Muscat..”
Dr. Constance LeBlanc, Halifax, Canada
“We owe you a very special “thank-you” for
being included in your
International Conference. Things were professionally organised, efficiently run
and we were made to feel welcome and appreciated. We particularly enjoyed the
audience participation in sessions...”
Prof. Anna Jarvis, Toronto, Canada
“I regularly speak at many conferences and
have seen great and not-so-great conferences. The conference in Oman last week
was definitely one of the great ones...”
Dr. Russell MacDonald, Toronto, Canada
We hope that the third international
conference will maintain the high standard achieved.
Below are some of the important abstracts
of talks presented by international speakers during the conference.
Speaker: Dr. J. Scott Delaney, McGill
University, Montreal, Quebec, Canada
Dr. Delaney practices emergency medicine
and sport medicine at McGill University in Montreal, Quebec. He has a
fellowship in sport medicine and is the research director for the McGill
University Health Centre Adult Emergency Department. He is an assistant professor
at McGill University and is the team physician for the local professional and
university football and soccer teams as well as Cirque du Soleil. He is a
member of the editorial board for the Clinical Journal of Sport Medicine. He
has supervised numerous Middle East emergency medicine residents and three
Omani sport medicine fellows at McGill University.
Cauda Equina Syndrome
Cauda Equina syndrome
(CES) is a serious cause of back pain which, if missed, can cause permanent
disability including lower extremity paralysis, sexual dysfunction and loss of
bowel/bladder control. CES is an acute stenosis of
the lumbar spinal canal leading to compression of neural elements below the
first lumbar segment (L1). Compression of the cauda equina can occur rapidly in the case of fractures, acute
disc herniations and spinal haemorrhage. It can occur
more insidiously in cases of tumour compression or spinal abscesses and often,
symptoms of pain, numbness or weakness are present or progress over a period of
weeks. It is in these insidious situations where the emergency physician can
make an early diagnosis and prevent lifelong morbidity in a patient.
The so called “red flags” on history and
physical examination for patients with back pain include many of the clinical
features of CES and emergency physicians should be familiar with these. CES
will present with bilateral neurologic findings, although physicians should not
be fooled as the findings are not usually equal bilaterally. CES will
classically present with saddle anaesthesia, urinary retention, fecal incontinence and decreased or absent anal tone. The
urinary incontinence of CES is actually over flow incontinence. An early sign
of compression of the sacral nerves in CES is a residual bladder volume of more
than 150 cc after a patient has voided completely. This can be measured by
bladder scan or bladder catheter insertion.
The need to diagnose CES early is vital as
the biggest predicator of final neurologic disability is the amount of
neurologic disability at the time of treatment. When CES is suspected, the
spinal canal must be visualized as quickly as possible. The spinal canal can
only be visualized by a MRI, CT myelogram or a plain myelogram. Plain X-rays or a plain CT scan of the lumbar
spine will not adequately visualize the spinal canal and can be normal in cases
of severe spinal canal compression. When imaging the spinal canal for possible
CES, the entire spine should be visualized as up to 10% of patients with
tumours will have silent metastasis in another spinal location. When the
diagnosis of CES is confirmed or seriously suspected, the emergency physicians
should begin treatment with analgesics, antibiotics in possible infectious
related compressions and dexamethasone in tumour
related compressions. The emergency physician should speak with the consulting
spine surgeon regarding the choice of antibiotics and dose of dexamethasone, as doses ranging from 4mg to 100mg of dexamethasone for tumour related compression have been
described in the literature. Definitive treatment may involve radiation,
especially for tumour related compression that involves several levels of the
spine, or surgical decompression for an abscess or single level of tumour
related compression.
Updates in Concussions
There have been several changes in the
diagnosis and management of concussions in recent years. A concussion is now
defined as any alteration in cerebral function caused by a direct or indirect
(rotation) force transmitted to the head resulting in one or more of the
following acute signs or symptoms: headache, confusion/disorientation, loss of
consciousness, light sensitivity/photophobia, nausea/vomiting, abnormal vision,
dizziness/vertigo, hearing problems and memory difficulties. The onset of signs
or symptoms may not be immediate and can occasionally take hours to develop.
Other complaints which are not usually immediately evident may also include:
sleep irregularities, fatigue, personality change, lethargy and depression.
Physicians and nurses should be aware that it is not
necessary to have suffered a loss of consciousness to have a concussion, nor is
it always necessary to have suffered a direct blow to the head. Any fall,
tackle or car accident which causes the head to accelerate or decelerate
quickly can cause a concussion.
It is not compulsory to have advanced
imaging for the diagnosis of a concussion. In the vast majority of patients
with a concussion, brain CT scans and MRI’s are normal. Physicians can feel
confident that if a patient has a normal neurological examination with mild
and/or improving symptoms, brain imaging is not necessary. Perhaps the most
sensitive tool for making the diagnosis of a concussion is the patient’s
symptoms. Using a patient’s symptoms to diagnose and follow recovery from a
concussion has been proven to be even more sensitive than special
neuropsychological testing. Very often these symptoms will worsen with physical
exercise or cognitive stress (ex. doing school work). Treatment of a concussion
most importantly involves stopping or avoiding activities or scenarios which
exacerbate or cause symptoms to recur. While physical exercise and cognitive
stress have been mentioned, others irritants may include exposure to bright
lights, loud noises, working on a computer, etc. It is believed that continuing
to exacerbate concussion symptoms only delays healing. While headaches are the
most common symptom of a concussion, they can be very difficult to treat with
medication. In fact, the use of medication is often used as a diagnostic tool,
in that; if medication relieves the headache, then the headaches were probably
not concussion related. Patients who are still experiencing symptoms from a
concussion are believed to have a lower threshold and be more at risk for
another concussion. No patient should be allowed to return to an environment
where another blow to the head or acceleration/deceleration of the head can
occur (ex. sports) until all of their symptoms have resolved. Full resolution
of symptoms requires that a patient is asymptomatic at rest and after a trial
of exercise or exertion.
Cervical Spine Immobilization and Log
Rolling
Immobilization and movement of a patient
with a potential cervical spine injury are important skills for emergency
physicians and nurses to possess. Indications for immobilization of a patient’s
cervical spine are similar to the NEXUS criteria for imaging a cervical spine
and include a known or possible history of trauma and one of the following:
posterior midline cervical spine bony tenderness, neck stiffenss,
altered level of consiousness, focal neurological
deficit or a painful distracting injury. Immobilization of the cervical spine
should be maintained in the midline position or the position of comfort if a
patient is unable to return the cervical spine to a neutral position due to
pain or neurologic symptoms caused by movement of the neck. Immobilization does
not include traction of the cervical spine and is best accomplished with the
patient in a supine position. While different techniques exist, perhaps the
most sturdy and secure position involves the person controlling the head and
neck (called the immobilizer) standing or kneeling at the head of the patient
and grasping both trapezii with their hands while
nestling the patient’s cervical spine and head in between the immobilizer’s two
forearms. This procedure can be accomplished both with and without a hard
cervical collar in place.
Often a patient with a potential cervical
spine injury must be turned or ‘log rolled” onto their side to allow for
inspection of the back area or placement of a spinal board. The emergency
physician or nurse should control the situation and arrange as much help as is
needed. Ideally, the physician or nurse may remain free of duties to control
airway issues if they arise. If there are enough people to help turn the
patient into a supine position, there is one person controlling the head and
neck, one person at the shoulders, one person at the hips, one person at the
feet and one person to place a rigid spine board if needed. The people controlling
the shoulders and hips should cross arms across the patient so they move more
as a single unit. The immobilizer will lead the team by explaining the
procedure to the patient and other team members. The immobilizer coordinates
all movements with commands and other team members do not initiate movement of
the patient until the immobilizer directs such movement. If a patient is not
supine, the patient will need to be placed in a supine position for better
evaluation and potential airway control. In a patient who is prone or on his or
her side either in the emergency department or at the scene of injury, the
immobilizer should begin with his or her hands in the final position desired
(with the thumbs facing upwards) and then turn them back into the patient. Working
the hands “backwards” by starting in the ideal final position and turning them
back into the patient will avoid awkward twisting of the immobilizer’s arms
when rolling the patient into a supine position.
In an emergency situation where the
physician or nurse is alone with a patient who has a potential cervical spine
injury who must be rapidly turned onto his or her side, the procedure can be
performed with one person. The immobilizer grasps one side of the trapezius with his or her hand and nestles the patient’s
head and neck against their forearm. The other arm is used to grasp the
shoulder or clothing of the patient and pull them onto their side at ninety
degrees. The patient should be turned towards the side that the immobilizer has
grasped the trapezius so that as the patient is
turned, the head and neck can lean against the immobilizer’s forearm and remain
in a neutral position. The immobilizer then places a knee behind the patient to
prevent the patient from rolling back into the supine position. In this final
position, one of the immobilizer’s hands and forearms is used to hold the head
and cervical spine in a neutral position, the other hand is free to help
control the airway (ex. remove vomitus) while one of
the immobilizer’s knees is against the patient’s back preventing them from
rolling back into a supine position.
Mass Casualties and Triage
A disaster is defined as any multiple
casualty incident which overwhelms the response
capabilities of the available resources. Several injured patients may overwhelm
a single physician, while it may take dozens or hundreds of patients to
overwhelm an event with greater resources. In a disaster situation, the
physician’s goal is to try and save as many lives as possible. In some extreme
instances, care should be withheld from severely injured patients so that
limited resources are available for others. Perhaps the most important decision
to be taken in such a situation is for the physician to recognize that they are
in a disaster scenario and realize that assessment and treatment principles are
now different from the regular practice in the emergency department.
While there are several simple triage
systems, the START (Simple Triage and Rapid Treatment) system using the
assessment of RPM’s (Respirations, Perfusion, Mental status) is a popular system to use. Physicians should
become familiar with one system. Most systems will colour code or give a
numerical value to each patient. After quick assessment, injured patients
should be placed in one of the four colour groups depending on the severity of
their injuries.
|
Color |
Priority |
Description |
Red
|
1 |
May survive if given immediate
simple life saving measures |
Yellow
|
2 |
Should survive if given care
within a few hours |
|
Green |
3 |
Walking wounded: minor injuries
that do not require rapid care |
Black
|
4 |
Deceased or severely injured
patients unlikely to survive |
Initially, all patients who can walk are
asked to leave the immediate scene. Next, the physician should move quickly to
individual patients, assessing respirations, circulation status, and mental
status. Under true disaster situations, CPR should not be performed during
triage. As the physician moves from patient to patient, the most aggressive
measures that should be performed include basic airway opening maneuvers and applying direct pressure over an obvious
external source of bleeding. Basic airway maneuvers
in a disaster triage situation should be limited to simple procedures such a
clearing the airway and performing a chin lift or jaw thrust. Once an initial
triage has been completed, the physician may then begin more definitive care
for patients. Depending on the resources available, this may involve Basic Life
Support maneuvers such as opening and maintaining an
airway or compressing an actively bleeding wound. Advanced Trauma Life Support
measures, such as performing a needle decompression of a suspected tension pneumothorax, may also be required. The physician should
also remember that it is critical that the patients be reassessed and retriaged. Triage is designed to be a dynamic process as
patients who seemed well can decompensate and change to a more serious
category. By reassessing patients, the physician will be able to detect those
patients who may have deteriorated and who now may require immediate care.
Two situations which may affect triage include lightning injuries and
blast injuries. In a lightning injury, a large amount of DC current, often more
than 1,000,000 volts, is delivered to a patient. This can result in respiratory
arrest from a CNS insult and asystole from the DC
current delivered to the cardiac tissue. If the patient’s respiratory status
can be supported, often the CNS will recover and reinitiate respirations. If
the circulatory system can be supported with cardiopulmonary resuscitation
(CPR), the intrinsic electrical activity of the heart may restart organized
cardiac contractions once again. As such, it is often said that triage
principles are reversed in lightning injuries, as the patients who appear dead
with no respirations and pulse are the ones who are treated aggressively first.
In a blast injury from an explosion, many patients near the blast may suffer
ruptured tympanic membranes. Patients may be unable to hear because of this
injury, may not respond to verbal questioning and may be mistaken for a
confused patient. This may lead to inappropriate triage and patient disposition
if the physician is not aware of this common injury.
Speaker: Dr. Constance LeBlanc, Dalhousie
University, Halifax, Nova Scotia, Canada
Dr. LeBlanc is a medical graduate of Université Laval in Québec, Canada. She is an Associate
Professor of Emergency Medicine at Dalhousie University where she served as
Emergency Medicine program director for the CCFP(EM)
from 1997 until 2005. She holds a Master of Arts in Education degree from Mount
Saint Vincent University in Halifax. Connie has served as chair of Continuing
Medical Education for the Canadian Association of Emergency Physicians (CAEP)
since 2003 and as faculty member for the CAEP roadshow
on education “ED STAT!” since 2004. She also serves as a medical control
physician for both Life-flight Nova Scotia and for the Nova Scotia Poison
Information Center.
Medical Education Track
Effective clinical teaching and the
provision of effective feedback are key elements in training insightful
physicians and the cornerstone of clinical medical education. We know that
learning and the development of insight is facilitated by the provision of high
quality feedback yet, we struggle to achieve the honesty to optimize this
process for our learners. Why is honesty so difficult?
Bandiera et al. have identified some core behaviors in outstanding teachers; many
of these skills can be learned. Students
appreciate enthusiasm, interest in them as people first and clinical teaching
including orientation and appropriate feedback among these valued qualities.
There has been a notable increase in
literature in the past decade in teaching in ambulatory settings and in
Emergency Medicine where there was a paucity
previously.
References
1. Irby, D.M., Teaching and Learning in Ambulatory Care Settings: A
Thematic Review of the Literature. Acad Med, 1995.
70(10): p. 898-931.
2. *Review of what expert Canadian ED teachers have to say about teaching
in the ED. Outlines twelve strategies for success.
3. Heidenreich, C., P. Lye, D. Simpson, and M. Lourich. The Search for Effective and Efficient Ambulatory
Teaching Methods Through the Literature. Pediatrics, 2000. 105(1): p. 231-237.
4. Irby Dm. Teaching and learning in ambulatory care settings: A thematic
review of the literature. Acad. Med.1995;70(10):898-931
5. *Excellent systematic review (1980-1994) of effective ambulatory
teaching methods.
6. Lipsky, M., C. Taylor, and R. Schnuth,
Microskills for students: Twelve tips for improving
learning in the ambulatory setting. Medical Teacher, 1999. 21(5): p. 469-472.
7. Sherbino, J, Frank, J. Lee, C.and
Bandiera, G. Evaluating “ED STAT!”:
A Novel and Effective Faculty Development Program to Improve Emergency
Department Teaching. Acad. Emerg. Med. 2006,13(10): 1062-1069.
8. Thurgur L, Bandiera G(SRA), Lee S, Tiberius R. What emergency medicine learners
wish their teachers knew. Academic Emergency Medicine. 2005. 12:856-861.
Orienting the Learner in Medical Education
At the outset of the shift or rotation it
is essential to set the foundations for learning and clarify the expectations
of both the trainee and the teacher. Although orienting learners seems time-consuming,
taking time to orient the learner will this will pay off time and again
throughout the rotation or the shift. It is important to take a few moments to
acquaint yourself with the learner at the beginning of each rotation. From a
student’s perspective, this demonstrates your interest in them and their
learning. From a preceptor’s perspective, this provides you with essential
information about the learner that will facilitate discussing patient
presentations, differential diagnoses and allow you to make informed choices
for feedback. Data collected at orientation will also serve to inform the
teacher about the level of supervision that will be required to provide safe
care.
Ascertain the level of training, previous
fields of training, rotation-specific experience, focused interests (program of
training and specific goals) and skill level (experience in this area of
medicine) of the learner working with you. This provides important information
for the degree of supervision required, the depth of teaching and questioning,
focuses the teaching you will provide and general management strategies with
regards to time and supervision for the rotation. It is also very important at
this time, to state that you will provide feedback to facilitate their learning
and that the intention is so doing is to optimize learning rather than to be
critical of their performance. The feedback and supervision processes will be
facilitated by the information gathered during orientation. The fact that a
preceptor has shown interest in the learner and their objectives will also help
focus feedback in the most important areas for that learner.
Orienting the learner should take 10
minutes at the outset of the rotation; time saved in doing so will be far
greater that 10 minutes the vast majority of the time. A brief orientation at
the outset of each shift serves to further convey these messages and ensure
open communication.
Teaching is challenging due to competing
needs: those of patients and their families, needs of learners, team members,
administrative or patient flow requirements in addition to your personal
learning needs. Balance in multitasking these is essential to providing
learners with meaningful learning experiences, while providing timely and high
quality care for patients. In order to teach effectively and efficiently, it is
important to identify any specific issues that will affect these aspects of our
work.
References
1. Bandiera Glen, Blouin Danielle, Frank Jason R,
LeBlanc, C., Lee Shirley, Nuth Janet, & Sherbino Jonathan. E.D. STAT Strategies for Teaching
Anytime. 2007. 2005. Ref Type Catalogue
2. Bandiera G, Lee S, Tiberius R. Creating effective
learning in today’s emergency departments: How accomplished teachers get it
done. Ann Emerg. Med. 2005:45:3:253-261.
3. Irby D. What clinical teachers in medicine
need to know. Acad. Med. 1994;69(5):333.
*A comprehensive review of how ambulatory medicine changed medical
education and how to meet the challenges of teaching different learners in a
high-pace environment.
Feedback in Medical
Education
During orientation, educators should include a plan
for feedback and for the educational experience to learners. This sets the
stage for feedback to be received favourably and normalizes it. Feedback is the
most common teaching tool discussed (and studied) in the literature. It differs
from evaluation in that it is performance based rather than comparative.
Feedback can be part of evaluation, but the reverse is not true. Feeding back
data on the many facets of clinical performance should be continuous whereas,
rating learners comparatively to their peers or to practicing clinicians
continuously is futile and could serve to discourage the learner who is
struggling rather than providing a plan or tools to get caught up. The key role
of feedback is clearly outlined in the following quote:
“self-assessment,
knowing one’s limits, and knowing when to seek help depend upon feedback”.
Without feedback, students’ mistakes will
persist and their positive attributes may wane from lack of reinforcement. The
provision of high quality feedback is central to student learning. Throughout
the literature, the features of good feedback vary little. The same features
surface time and again, sometimes with different labels. (See Figure 1 below)
Figure 1: Features of Proper feedback:
1. Elicit self-assessment (determine the degree of insight)
2. Say that it’s feedback (label it)
3. Contextual (for today’s patients…)
4. Immediate, timely (after the case, after the day…)
5. Based on objective information (examples of occurrence(s), direct
observation)
6. Discuss the behaviour- not the person
7. Start with positive and move to negative and end on positive (the
knuckle sandwich)
8. Constructive with a plan for corrective measures
9. One on one (privacy)
10. Not evaluation (no judgment, no rating)
11. Solicit feedback on the feedback
12. Solicit feedback on teaching
A more detailed explanation of the above
characteristics is provided in the following corresponding bullets.
1. The process of having the learner self-assess serves two purposes:
firstly to determine their degree of insight, and secondly to foster reflection
on the events of a shift.
2. Often learners don’t recognize that they are receiving feedback. Labeling it is important to draw attention to its
importance to us and to the student.
3. It is not imperative to provide feedback on everything in one shift.
Metered doses of feedback will be better received and allows the preceptor to
triage the feedback according to the specific needs of each learner.
4. The best feedback is delivered immediately. Like reprimanding a pet!
5. Feedback on weak data will not be taken as seriously as that on directly
observed or clinically rechecked information.
6. Opening with a positive comment is easier for us and closing with one is
easier for the learner. This is not true for all situations.
7.
Commenting on the
length of a student’s arms is not helpful, suggestion or recommending the use
of a stool for a procedure is.
8. Make every attempt to identify a trend or thread for feedback for the
entire shift such as: broader differentials are required, or more detailed
histories are necessary.
9. Providing all feedback in relative privacy will reduce the “special
attention” for weaker students that all ED staff recognize and remember.
10. Daily evaluations are not helpful or constructive, especially for those
learners who are behind their peers. Feedback will provide a better learning
experience with an evaluation every 2-3 weeks to document their progress.
11. Ask learners if they are confident they know what to change and if they
are comfortable with the information provided.
12. Ask them too, how you could improve the experience for them.
Medical educators must strive to follow the
principles of good feedback in all of our student interactions. Fear of tears,
anger, accusations, poor teaching evaluations and lack of time are all barriers
to the provision of honest feedback. Overall, students rate preceptors who
provide honest feedback more highly than their peers.
Ask yourself if a low threshold to tears
should allow a trainee to move forward uncorrected. Would you appreciate your
son or daughter not receiving the feedback they require to improve in this
situation? Do not learners from minority groups deserve the same learning
opportunity as others? Why should we remain silent about breaches in
professionalism when all available data state that most patient complaints
about physicians fall in this sphere of their practice?
This is important and we owe it to all our
learners to provide them with this key information to further their development
and to assist them in developing insight and reflective practices that will
last throughout their careers. Skill in providing honest feedback effectively,
like other skills, takes practice and will improve over time.
References
1. Ende J, Feedback in Medical Education. JAMA 1983;250(6): 777-781.
2. Epstein R, Assessment in Medical Education, N Engl
J Med 2007 Jan25,356(4):387-96. Review.
3. Eva, K.W. and Regehr, G. (2005).
Self-assessment in the health professions: A Reformulation and Research Agenda.
Academic Medicine, 80(10 Suppl): S46-S54.
4. Feedback. The Foundation for Medical Practice Education. Education
Module. McMaster University; 11(special issue, April 2003).
5. *Booklet published at McMaster University through the foundation for
continuing medical education. Nice review.
6. Kruger, J. and Dunning D. (1999). Unskilled and unaware of it: How difficulties
in recognizing one’s own incompetence lead to inflated self-assessments.
Journal of Personality and Social Psychology. 77:1121-1134.
The Hidden Curriculum
The process of educating physicians is
complex and reaching far beyond the curricula set forth by medical schools,
despite written objectives and formal curricula, students learn far more than
we teach.(1) This gap is the hidden curriculum. (2)
In Posner’s description of the types of
curricula, he describes the following five: formal, informal, extra, null, and
hidden. (3) These are explained in Figure 2 Below.
Figure 2: Posner’s Five Types of Curricula.
1. Formal: This includes information an institution would have in written
form for dissemination to the public including course prerequisites, requirements,
delivery and objectives in addition to the course objectives.
2. Informal: The educational programme as it actually is taught. Some
variation from the formal curriculum will occur in every programme.
3. Extra: This consists of all activities not part of the informal
curriculum but affiliated with the institution.
4. Null: This describes the elements with potential for inclusion in the
formal or the informal curricula, but omitted form both. The content of the
null curriculum can send important messages about values and socio-political
pressures in the institution and beyond.
5. Hidden: This includes a set of messages transmitted tacitly through
working with others and mostly includes social and ethical issues that surround
the education provided. These are far more pronounced in apprenticeship models.
The hidden curriculum can include things
from the car we drive to how we regard other team members and even patients.
The messages in the hidden curriculum are often remembered despite the
unintentional nature of their delivery to our students. (4) It will behoove all physician educators to maintain a certain
degree of “role-model consciousness”.(5,6,7) Derogatory comments about
patients, their families, team members or other physicians are inappropriate
and we should refrain from entering or engaging in discussions of this type
while teaching trainees at any level. These comments and attitudes have been
shown to be exceptionally stressful to junior learners. Expressing frustrations
about our area of specialty, burnout, finances, are inappropriate unless there
is a specific question in this area.
Hopefully, we will behave as if others are
watching to benefit from the Hawthorne effect improving the quality of our role
modeling and perhaps our patient care. (8,9)
References
1.Hafferty F.W. Into the
Valley: death and the socialization of medical students. Yale University Press,
1991.
2. LeBlanc C. Exploring the Hidden Curriculum in Emergency Medicine.
Masters’ thesis for Master of Arts in Education degree. Mount Saint Vincent
University. Canadian thesis site.
3.Posner G. Analyzing the Curriculum. 2nd ed. McGraw-Hill, 1995.
4.Wong JG. Promoting professionalism. Are we living by the values we
expect from our students? Postgrad Med 1999;
106(5):11-2, 15.
5.Patenaude J, Niyonsenga T,
Fafard D. Changes in students’ moral development
during medical school: a cohort study. CMAJ 2003; 168(7):840-844.
6.Hicks LK, Lin Y, Robertson DW, Robinson DL, Woodrow SI. Understanding
the clinical dilemmas that shape medical students’ ethical development:
questionnaire survey and focus group study. BMJ 2001; 322(7288):709-710.
7.Kenny NP, Mann KV, MacLeod H. Role modeling in physicians’ professional formation:
reconsidering an essential but untapped educational strategy. Acad Med 2003; 78(12):1203-1210.
8.Coulehan J, Williams PC. Vanquishing Virtue: The
Impact of Medical Education. Acad Med 2001;
76(6):598-605.
9. LeBlanc C, and Heyworth J. Burned out or fired
up? CJEM, March 2008.
* Article on the lack of evidence for
burnout among Emergency Physicians attending the ICEM 2006.
Informed Use of Cardiac Markers in the
Emergency Department
Chest pain is a common Emergency Department
(ED) presenting complaint. We know from the medical literature that we miss
between 2-5% of Acute Myocardial Infarct (AMI) in the ED. (1) We can ill afford
to miss this important diagnosis, however, time, money and space issues do not
allow for routine admission of all patients with chest pain.(2) Risk
stratification is required.
The 3 key elements of risk stratification
for patients with chest pain for whom we are considering an ACS (Acute Coronary
Syndrome) these include: clinical assessment electrocardiogram (ECG) and
cardiac markers. (3,4,5)
Three main take home points include:
1. If a patient has a positive ECG, the time to decision should be less
than 10 minutes. Thrombolytic therapy should be given if the time to access a
cardiac cathreterisation laboratory is greater than
90 minutes in the absence of contraindications, otherwise the latter should be
the primary intervention.
a. Either troponin-T or troponin-I
should be the assay of choice for a single institution. A single test is only
acceptable for patients with chest pain lasting for greater than 20 minutes.
(6)
b. To effectively use either troponin assay in
the ED, the pain must have been present for at least 20 minutes and the test
must be repeated at least 10 hours after the pain started to achieve an
excellent sensitivity.
c. Serial ECGs and 15 lead ECGs
are very useful in the setting of acute chest pain and are underutilised.
Risk stratification of patients presenting to the ED
with chest pain will be more effective with use of serial ECGs
and extended ECGs and appropriate use of cardiac
markers.
References
1. Pope H et al. Missed Cardiac Ischemia in the Emergency Department.
Circulation. 2000; 342(16):1163-1170
2. Christenson J. et al. A Clinical Prediction Rule for Early Discharge of
Patients With Chest Pain. Ann Emerg
Med 2006; 47(1):1-9.
3. Ohman E et al. Cardiac troponin T
levels for risk stratification in acute myocardial ischemia. NEJM 1996;
335(18):1333-1341.
4. Babuin L and Jaffe A. Troponin:
the biomarker of choice for the detection of cardiac injury. CMAJ 2005;173(10):1191-1202.
5. Guo X et al. The predictive value of the bedside troponin T test for patients with acute chest pain. Clin Card 2006:11(4):298-301.
6. Mair J et al. Equivalent Early Sensitivities of Myoglobin, Creatinine Kinase MB Mass, Creatinine Kinase Isoform Ratios, and
Cardiac Troponins I and T for Acute Myocardial
Infarction. Clin Chem;41(9):1266-1272.
Speaker: Dr. Richard Verbeek,
University of Toronto, Toronto, Ontario, Canada
Dr. Verbeek is an
Assistant Professor in the Department of Medicine at the University of Toronto.
He is also a medical director at the Sunnybrook-Osler Centre for Prehospital Care where he provides oversight for the Toronto
EMS Paramedic Program, the largest municipally based EMS service in Canada
comprised of over 1000 paramedics. Dr. Verbeek
is currently the chair of the Prehospital Medical
Advisory Committee which is responsible for advising government on standards
for paramedic care in Ontario.
Integrating Paramedicine
into the Health Care System
Traditionally paramedicine
has developed by focusing on the continual expansion of a paramedic scope of
practice necessary to provide high level prehospital
care. This has led to EMS systems that can be somewhat isolated from the
general medical community. Overall little attention has been paid as to how paramedicine can be integrated into the overall health
care. Three examples were given of an integrated approach which has resulted in
impressive positive contributions to patient outcomes: trauma management,
management of acute ST-segment elevated myocardial infarction (STEMI) and acute
stroke management. Each of these systems is characterized by the development of
hospital-based centers of excellence and the
following administrative approach:
•
Planned integration of EMS practice and
communications into a system wide plan
•
Triage protocols developed by EMS and
external consultants
•
Steering committee oversight that includes
EMS in a leadership role.
It is important to understand that these
programs have a huge impact on improving patient care without the need to
increase the scope of practice for paramedics. The important factor is the
system approach and to understand that EMS is less effective when not
integrated into the health care community. Medical leadership at this level is
characterized by EMS physicians who have a focus on development of EMS systems
of care in addition to the emergency care of individual patients.
References
1. Trauma care regionalization: a
process-outcome evaluation. Sampalis JS, Denis R,
Lavoie A, Fréchette P, Boukas
S, Nikolis A, Benoit D, Fleiszer
D, Brown R, Churchill-Smith M, Mulder D. J Trauma.
1999 Apr;46(4):565-79; discussion 579-81
2. A citywide protocol for primary PCI in
ST-segment elevation myocardial infarction. Le May MR, So DY, Dionne R, Glover
CA, Froeschl MP, Wells GA, Davies RF, Sherrard HL, Maloney J, Marquis JF, O’Brien ER, Trickett J, Poirier P, Ryan SC, Ha A, Joseph PG, Labinaz M. N Engl J Med. 2008 Jan
17;358(3):231-40.
Overview of paramedic scope of practice
Scope of practice for all health care
professionals including paramedics is a simple list of procedures and
medications that can be provided by an individual with specific credentials.
Scope of practice is not an educational curriculum or an educational standard.
Nor is scope of practice is a profile of clinical competencies or a clinical
standard of care. However a well defined scope of practice is needed to guide
the development of these aspects of training and performance. There can be
various levels of scope of practice for paramedics with varying degrees of
training. It is desirable that these levels be limited to no more than four or
five since this facilitates job portability, efficiency in training programs
over wide geographic areas and “branding” of paramedicine
which is essential for public understanding and acceptance. Many systems
operate very successfully with only one level of paramedic scope of practice.
There are four essential features that must
be met before a paramedic can be authorized to perform a defined scope of
practice. These are:
•
Education
–
Acquisition of knowledge, attitudes,
psychomotor, and critical decision making competencies
•
Certification
–
Formal verification that competencies have
been achieved via an exam process
•
Licensure
–
Legal authorization to perform a defined
scope of practice
•
Credentialing
–
Local authorization to perform a defined
scope of practice (usually by medical director)
Internationally, many different countries
use different terms to describe prehospital care
providers with similar scopes of practice. Alternatively, the same term (e.g.
“paramedic”) can mean a very different scope of practice from country to
country. Therefore is it essential when having a discussion with an
international colleague to define what scope of practice applies to any
particular term used to describe prehospital care
providers.
References
1. Canada – Paramedic Association of Canada –
www.paramedic.ca - National Occupational Competency Profile
2. USA - National Highway Traffic Safety
Administration - www.nhtsa.dot.gov – Scope of Practice Model
3. Ireland - Prehospital
Emergency Care Council – www. phecc.ie – Education
Training and Standards
4. Australia
http://en.wikipedia.org/wiki/Paramedics_in_Australia
Challenges of EMS Research
EMS is a very challenging environment in
which to conduct clinical research. Nevertheless clinical research is required
since what works in emergency medicine is not guaranteed to work in paramedicine. Controversies that have recently arisen are
the benefit of prehospital ACLS, ATLS, pediatric intubation and intubation of patients with severe
head injuries. It is important for EMS medical directors to have a research
perspective during their daily work since there is much to be learned and
clinical research gives academic credibility to the practice of paramedicine.
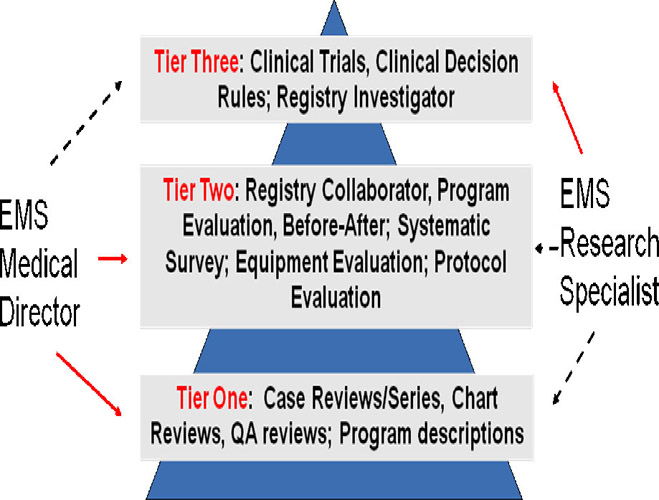 |
One approach to developing and maintain a
viable and worthwhile EMS research program is to understand the different roles
of an EMS Medical Director and an EMS research specialist whose sole focus is
clinical research. Three tiers of clinical research projects are proposed as
outlined below. These tiers highlight where an EMS medical director and an EMS
research specialist can most productively focus their efforts. The solid lines
represent types of research that can be undertaken as the principle investigator
while the dashed lines represent areas for collaboration.
This allows a EMS
medical director who is often fully occupied with their administrative role to
contribute important new knowledge to the scientific literature while ensuring
that are not overwhelmed with research responsibilities. Several examples were
presented of research that has been published by the University of Toronto in
each of these tiers over the past 5 years either by the EMS medical director or
the EMS research specialist.
References
One approach to developing and maintain a viable
and worthwhile EMS research program is to understand the different roles of an
EMS Medical Director and an EMS research specialist whose sole focus is clinical
research. Three tiers of clinical research projects are proposed as outlined
below. These tiers highlight where an EMS medical director and an EMS research
specialist can most productively focus their efforts. The solid lines represent
types of research that can be undertaken as the principle investigator while the
dashed lines represent areas for collaboration.
This allows nt:
major-bidi;mso-ansi-language:EN-GB'>www.rescu.net (Website of University of
Toronto EMS research program).
Speaker: Dr. Patrick Melanson,
McGill University, Montreal, Quebec, Canada
Dr. Patrick Melanson
is an emergency physician at the McGill University Health Centre. He also has a
fellowship in critical care medicine and he practices both specialties at the
Royal Victoria hospital. He is the program director of the critical care fellowship
at McGill University.
Diagnosis and Management of Shock in the
Emergency Department: A Physiological Approach
Shock can be defined as an impairment of
tissue oxygenation and perfusion. The physiological determinants of oxygen
delivery are the cardiac output (CO), the blood haemoglobin concentration, and
the oxy- haemoglobin saturation. Relying on a systemic Blood Pressure (BP)
measurement as the primary indicator of shock is problematic since BP is often
maintained at normal values despite significant decreases in CO due to
compensatory vasoconstriction and tachycardia. Hence, some measure of the
adequacy of blood flow or systemic oxygen delivery such as serum lactate or
central venous oxygen saturation should be considered.
However, knowledge of the physiologic
determinants of BP (BP= C.O. x SVR) is useful when attempting to classify shock
to and develop a specific differential diagnosis and therapeutic plan. Shock
can be classified broadly into a PUMP problem, meaning a low cardiac output, or
an arterial TONE problem, meaning a low systemic vascular resistance.
Furthermore, PUMP problems can be further sub-classified into HYPOVOLEMIC shock
(low preload), CARDIOGENIC shock (decreased contractility), and OBSTRUCTIVE
shock (increased afterload).
The clinical exam is useful but not
completely reliable when attempting to classify shock. The pulse pressure
between systolic and diastolic reflects the cardiac stroke volume and the
diastolic pressure reflects the systemic vascular resistance. Patients with PUMP
problems typically have an elevated diastolic pressure (high SVR) and a narrow
pulse pressure (low stroke volume) with cool extremities due to poor peripheral
perfusion and vasoconstriction. Patients with TONE problems generally have a
low diastolic pressure (low SVR) and a large pulse pressure (normal or high
stroke volumes) with warm well perfused extremities.
Since the clinical exam is not completely
reliable and often equivocal, it is frequently necessary to place a central
venous catheter to allow measurement of a central venous pressure (CVP) as a
surrogate of preload status. Central Venous Oxyhemoglobin
Saturation (CVO2) measurement can also be acquired from the central line and a
serum lactate can be measured. A low CVO2 or a high serum lactate indicate
inadequate peripheral oxygen delivery and thus are consistent with a PUMP
problem.
Vasoactive medications should be chosen based on the
desired hemodynamic effect and their pharmacological profile. The first line
treatment of a PUMP problem is usually fluid resuscitation. It is obvious that hypovolemic shock should be treated with volume, but cardiogenic shock and obstructive shock may respond to
volume as well. There is no evidence demonstrating superiority of colloids
versus crystalloids. Either are acceptable choices. Removal or treatment of the
obstruction is the definitive therapy for obstructive shock. When PUMP problems
are not fluid responsive, inotropic agents should be
started. Although several different agents could be chosen, Dobutamine,
a beta-agonist, is the most common inotropic agent
used in the emergency department. Generally dobutamine
should be increased in a stepwise fashion until the CVO2 is greater than 70%.
For arterial tone problems, fluid
resuscitation is again the usual first line therapy. Those patients who are not
fluid responsive should have a vasopressor agent
started. The most common vasopressor used in the
emergency department is norepineprine (NE). NE is a
vascular alpha receptor agonist. Norepinephrine
should be increased in a titrated fashion until the Mean Arterial Pressure is
maintained at greater than 65 mmHg for the average patient.
Mechanical Ventilation in the Emergency
Department: The Basics
Patients requiring mechanical ventilation
in the Emergency Department can be classified into four physiological
categories; 1) Type 1 (hypoxemic) respiratory failure, 2) Type 2 (hypercapnic) respiratory failure, 3) Patients with normal
gas exchange and lung mechanics requiring intubation for non-pulmonary reasons
such as coma, and 4) Restrictive lung disease. The goals of mechanical
ventilation are to improve oxygenation, to improve ventilation, or to decrease
the work of breathing.
Although there are many different modes of
mechanical ventilation, Assist-Control (A/C) also known as Continuous Mandatory
Ventilation (CMV) is the preferred mode for the majority of patients requiring
initiation of mechanical ventilation in the ED. With A/C ventilation the
patient is able to trigger a machine breath by initiating a respiratory effort
which will be sensed as either a pressure drop or a change in flow by the
machine. The ventilator will then deliver a set tidal volume before it cycles
off and allows exhalation to occur. In addition, if the patient is unable to
initiate a breath due to sedation, neuromuscular blockade, or some other
reason, a ventilator timer will ensure that a minimum set number of breaths
will be delivered each minute. Thus a minimum minute
ventilation is ensured.
When initiating mechanical ventilation on
most ED patients, the initial ventilator settings should be Assist Control mode
with a FiO2 of 100%, a PEEP of 5 cmH2O, a tidal volume of 8-10 ml/kg, and a
respiratory rate adjusted to target a normal PCO2 and pH (usually 10 -14
breaths per minute). The PEEP can be adjusted upward to improve oxygenation if
necessary. Otherwise, the FiO2 can be titrated downwards to maintain the Pulse Oximeter oxygen saturation above 90%.
Patients with hypercapnic
respiratory failure often have severe obstructive lung disease which limits
their rate of exhalation. If the next ventilator breath is initiated before
exhalation is completed, mechanical ventilation can lead to gas trapping,
dynamic hyperinflation, or Auto-PEEP. Auto-PEEP can cause decreased venous
return to the heart and severe hypotension or even a PEA (pulseless
electrical activity) cardiac arrest. If hypotension secondary to Auto-PEEP is
suspected, then the patient should be disconnected from the ventilator to allow
for complete exhalation of trapped air from the lungs and thus an improvement
in the BP. Once reconnected to the ventilator, measures should be taken to
decrease the chance that auto-PEEP will recur. These include increasing the
flow rate to decrease the Inspiratory/Expiratory
ratio, decreasing the tidal volume, decreasing the set ventilator PEEP, or
decreasing the respiratory rate. It may be necessary to accept hypercapnia (permissive hypercapnia)
in order to avoid auto-PEEP.
Another situation where permissive hypercapnia may be accepted is severe hypoxemic respiratory
failure secondary to ARDS. These patients often have severe atelectasis
and lung collapse with much smaller lung volumes than normal. Mechanical
ventilation with tidal volumes of 10-12 ml/kg may lead to an overstretch injury
to the lungs or “volutrauma”. Current recommendations
are to ventilate with tidal volumes of 6 ml/kg of predicted body weight. The
ventilator plateau pressure should be kept below 30 -32 cm H2O as well to
minimize the risk of barotraumas.
The ABC`s of
Sepsis: A Sepsis “Care Bundle” based on the Surviving Sepsis Campaign 2008
The components of the “Surviving Sepsis
Campaign: International Guidelines for the Management of Severe Sepsis and
Septic Shock:2008” most relevant to the emergency physician can be summarized
with an “ABC pneumonic” as a memory aid or care bundle for the EP faced with a
patient with severe sepsis or septic shock. The GRADE system was used to
evaluate the quality of evidence and the strength of recommendation. A strong
recommendation was graded as 1 and a weak recommendation as 2. The quality of
evidence was graded from high (A) to very low (D).
A) AIRWAY
·
Consider early use of
Non-Invasive Ventilator Support (BiPAP)
·
High risk intubation’s due to significant
incidence of peri-intubation hemodynamic
instability/severe hypotension. Use lower doses of your usual induction agents
(midazolam, fentanyl, propafol, etc.) or consider using etomidate
or ketamine which may cause less hypotension. Have phenylephrine or ephedrine drawn up in a syringe to respond
to hypotension immediately.
ANTIBIOTICS
·
Begin antibiotic therapy as early as
possible and always within one hour of diagnosing severe sepsis (1D) or septic
shock (1B).
·
Initiate broad spectrum therapy with one or
more agents active against the most likely pathogens (1B).
·
Panculture before starting antibiotics if time allows
(1C)
B) BREATHING
·
High incidence of Acute Lung Injury or
ARDS. Avoid over distension of lungs; If intubated,
use low tidal volume (low stretch approach) as per ARDSnet
study
·
Initiate Assist Control mode of ventilation
with initial tidal volume 8ml/Kg of Ideal Body Weight (reduce to 6 ml/Kg within
4 hours)
·
Maintain Plateau Pressure < 30 cm H2O
C) CIRCULATION
·
Begin resuscitation immediately in patients
with hypotension or lactate > 4mMol/L. Do not wait for ICU admission.
·
Measure serum lactate to assess for Global
Tissue Hypoxia.
·
Start Early Goal Directed Therapy (EGDT)
Protocol if any of the following criteria;
·
SBP < 90 mmHg after appropriate fluid
bolus
·
Lactate > 4 mmol/L
·
Evidence of one or more organ dysfunction
·
Patients with severe sepsis often require
large amounts of fluid during the first few hours. They often present with a
combination of dehydration, vasodilatation, venodilation,
and third space fluid losses due to increased microvascular
permeability, which are ongoing. One study demonstrated an average requirement
of 5 litres of crystalloid over the first six hours of therapy for patients
with severe sepsis.
·
Approximately 50% of patients with severe
sepsis will respond to fluids alone. It is reasonable to perform initial fluid
resuscitation targeting clinical endpoints such as HR, BP, and urine output.
·
Often patients with severe sepsis do not
receive enough fluids because of concerns about inducing pulmonary edema or volume overload in patients with previous
histories of CHF, low ejection fractions, or renal failure. Even in these
patient groups, outcome will be improved with aggressive fluid resuscitation
using appropriate resuscitation endpoints.
·
Initial minimum fluid bolus of 20 ml/Kg
crystalloid (or colloid equivalent) if sepsis induced hypotension. Use a fluid
challenge technique (1D). Administer fluid boluses (1000cc crystalloid or 500
cc colloid over 30 minutes) until CVP of 8 – 12 cm H2O (1D)
·
No clear advantage colloids versus
crystalloids.
·
Place central line (internal jugular or subclavian vein are the preferred sites) if the patient has
severe sepsis, sepsis-induced tissue hypoperfusion or
hypotension unresponsive to initial fluid bolus.
·
Early Goal Directed Therapy Resuscitation
Goals (1C)
·
CVP 8-12 cm H2O (1C)
·
MAP > 65 mmHg (1C)
·
CVO2 > 70 % (1C)
·
Decreasing lactate level
·
Repeat CVP and CVO2 measures q30 – 60
minutes.
·
If a central line cannot be placed and
CVP/CVO2 cannot be measured, then fluid therapy should be guided by repetitive
clinical assessments including determination of the JVP and auscultation of
lungs for rales/crackles. Clinical indicators of
adequate peripheral perfusion (i.e., mentation, urine
output, capillary refill, extremity warmth, etc.) should be reassessed
regularly.
·
Initiate vasopressor
therapy for persistent hypotension (MAP < 65 mmHg) despite adequate fluid
resuscitation. Maintain MAP > 65 mmHg (1C).
·
Vasopressors should be administered via a central
intravenous line. All patients requiring vasopressors
should have an arterial line placed as soon as resources allow. BP should be
monitored non-invasively at frequent intervals (i.e., q5min) until arterial
line monitoring is established (1D).
·
Norepinephrine at 2 – 50 mcg/min titrated upwards to keep
MAP > 65 OR
·
Dopamine 5 to 20 mcg/kg/min. (1C)
·
Consider PRBC transfusion if HBG < 10
g/L and patient has lactic acidosis, CVO2 saturation < 70%, acute
haemorrhage, or active coronary ischemia.
·
Consider inotropic
therapy if CVO2 remains below 70 % despite adequate fluid and PRBC
resuscitation (Dobutamine beginning at 2.5 mcg/kg/min
and titrating upwards q15min at 2.5 mcg/kg/min increments until CVO2 > 70%.
(1C)
D) DRUGS
·
Activated Protein C (rhAPC,
Drotecogin Alfa, Xigris)
·
Consider rhAPC
administration in collaboration with an Intensivist,
in adult patients with sepsis-induced organ dysfunction and a clinical assessment
of a high risk of death (two or more organ failure or APACHE II score >24)
and no significant bleeding risks/contraindications (2B).
E) ENDOCRINE
·
Corticosteroids –
·
Consider intravenous hydrocortisone for
adult septic shock when hypotension responds poorly to adequate fluid
resuscitation and vasopressors.(2C)
·
ACTH stimulation test no longer recommended
or required (2B)
·
Hydrocortisone is the preferred glucocorticoid (2B)
·
Hydrocortisone 50 mg iv q6h (if random cortisol done)
·
Also consider ‘stress dose’ steroids in the
absence of shock in patients with history of chronic steroid use or adrenal
insufficiency
·
Glucose control with insulin
·
Maintain blood glucose < 8.3 mmol/L with sliding scale subcutaneous insulin or insulin
drip protocol.(2C)
F) FIND
the source of infection and establish source control.
·
Perform focused clinical examination,
guided by risk factors.
·
Most likely sites are lungs (30%),
bloodstream (20%), abdomen (20%), and urinary tract.
·
Pan culture
·
At least 2 blood cultures prior to
antibiotic administration.
·
Cultures from other possible sites (sputum,
urine, CSF, etc.).
·
Directed radiological studies.
·
Source control measures (abscess drainage,
debridement of devitalized tissues, device or line removal) should be performed
as soon as possible.
Speaker: Dr. Anna-Maria Carvalho,
McGill University, Montreal, Quebec, Canada
Dr. Carvalho is
an Emergency Medicine specialist and a consultant in Aviation Medicine at
McGill University in Montreal, Canada. Her fellowship training in aviation
medicine encompassed fixed and rotor wing medical evacuations, commercial
aircraft health and safety, and occupational health of aircrew. She also
obtained her Flight Surgeon and Advanced Diving Medical Officer certificates
from the Canadian Armed Forces. Dr. Carvalho works as
a medical consultant to Air Canada, and as a flight physician for Skyservice Air Ambulance. She is assistant director for the
Aviation Medicine Fellowship program at McGill University and is course
director for Onboard Medical Emergencies, an annual educational event for
physicians.
Is your Emergency Department patient fit to
fly?
Certain chronic conditions, or
exacerbations of chronic disease, may make a person unfit to fly. Often, these
patients present to the Emergency Department. Knowledge of certain absolute and
relative contraindications to fly can make the travel experience safer for your
patient and reduce in-flight emergencies.
The commercial aircraft cabin (pressurized
to approximately 8000 feet above sea level) is hypoxic, compared to the ground
atmosphere. A normal, healthy adult will desaturate
to approximately 93%. Anyone with chronic lung disease, or with abnormal
sea-level saturation, will desaturate even further.
For this reason, anyone with an abnormal saturation at sea-level or with any
oxygen requirements at sea level will require oxygen in flight. This can be
arranged through the airline.
A passenger with anemia
(hemoglobin less than 90 g/L) cannot fly without
supplemental oxygen, even if the ground level saturation is adequate. The
decreased oxygen carrying capacity, in combination with the hypoxia of
altitude, will lead to decreased oxygen delivery to end-organs and may result
in a medical incident in-flight.
Often, emergency physicians encounter travelers who sustain a fracture requiring immobilization.
The usual risks of swelling are increased in the traveler
with a lower extremity fracture that cannot be elevated in the aircraft.
Furthermore, the hypoxic environment causes venodilation,
further increasing the risk of swelling and compartment syndrome. For this
reason, any cast less than 48 hours old must be bivalved
prior to flight. A safe alternative would be to splint the injured extremity.
Most major airlines have physicians on duty
to assist in making decisions regarding fitness to fly. Many airlines have a
medical form available on the company website to assist in identifying
potentially problematic medical conditions. Any uncertainty about prognosis for
travel should be cleared with the airline’s medical department.
Decompression Sickness
Sport diving continues to increase in
popularity, and Oman has developed into a dive destination, with various dive
shops and organized diving excursions in the Gulf of Oman. Emergency physicians
may encounter injured divers and must always consider the possibility of
decompression illness as the cause of the medical condition.
As a diver descends in the water, the
atmospheric pressure is increased, causing nitrogen to be dissolved into the
tissues (Henry’s Law). The longer the time under water and the deeper the dive,
the more the tissues will become saturated with nitrogen. When the diver
ascends, the nitrogen is off-gassed via the lungs. However, if the diver
ascends too quickly, or misses a decompression stop, the nitrogen will come out
of solution in the tissues. Depending on where the bubbles
are lodged, the diver may experience pain (joint, periarticular
tissues), shortness of breath (lungs), chest pain (lungs, heart), neurological
problems (spinal cord, brain), or vertigo (inner ear). Most symptoms
will occur shortly after the diver surfaces.
If the diver breath-holds while ascending,
the volume of air trapped in the lungs will increase in size (Boyle’s Law).
Eventually, air will be forced across the alveolar-capillary membrane or the
compliance of the alveoli will be overcome, causing rupture, resulting in one
of the pulmonary overpressurization syndromes, the
most serious of which is an arterial gas embolism (AGE). An AGE results when
air bubbles travel in the arterial circulation, resulting in obstruction of
blood flow.
Both decompression sickness (DCS) and AGE
are treated with recompression therapy in a hyperbaric chamber. Hyperbaric
therapy decreases the size of the bubbles and forces them back into solution,
until they can be off-gassed via the lungs. Transport of the injured diver to a
hyperbaric chamber must be done at sea level (to avoid further increase in the
size of the bubbles), with the diver on 100% oxygen.
In-flight Medical Emergencies
Increasing numbers of travelers,
longer flights, older travelers and travelers with undisclosed medical conditions all lead to
an increased number of in-flight medical emergencies across airlines around the
globe. The vast majority of in-flight emergencies fall into the categories of
neurologic complaints, gastrointestinal problems, respiratory difficulties, and
cardiac events. Flight attendants have limited first aid training and are not
equipped to deal with a major medical emergency. The health care professional
who volunteers to assist with an onboard medical emergency should be aware of
the resources available on, and the limitations of, a commercial aircraft.
Most major airlines subscribe to an online
ground support service, which provides medical assistance via satellite at any
time of day. The health care professional who assists onboard should ask the
flight attendant to contact the service, as an aviation medicine specialist can
assist in management of the ill passenger, and can advise on options for
diversion to a closer airport if necessary. Furthermore, many providers of this
type of service provide liability insurance for the health care professional
who volunteers to assist onboard the aircraft.
Most large aircraft will have an emergency
medical kit onboard. This is often locked, and can only be released to a
physician who can provide proof of licensure. The contents of the kit varies
from airline to airline, but should contain cardiac resuscitation medications,
antihistamines, antiemetics, bronchodilators,
glucose, and a minimum of 250cc of intravenous fluid. Medical equipment in the
kit includes a sphygmomanometer, a stethoscope, oral airways, and a setup for
delivering intravenous medications.
The aircraft will also carry a bag-valve
mask, an emergency oxygen tank and an automated external defibrillator, but
these are not included in the emergency medical kit. They are often located in
various storage spaces throughout the aircraft and must be requested
individually.
Despite best efforts, a death may occur in
flight. Should this happen, it is not a reason to divert the aircraft; the
flight should continue on to the scheduled destination. While a physician may
pronounce the death in-flight, the country of death will be at the next
landing.
The emergency medical kits include forms
for documenting the medical incident. These should be filled out, as with any
medical encounter, and a summary should be given to the passenger to present to
the physician who will be taking over care once on the ground.
Speaker:Dr. Peter Jones, Auckland, New Zealand
Dr. Jones has been a specialist in Emergency
Medicine since 2000. He has always been interested in good quality research
recently completed an MSc in Evidence Based Healthcare at Oxford University. He
is senior adjudicator for the Australasian College of Emergency Medicine
trainee’s Fellowship research papers and a member of both the ACEM Trainee
Research Committee and ACEM Clinical Trials Subcommitee.
He is organising the NZ arm of the multicentre Australasian Resuscitation in
Sepsis Evaluation trial. He has a wide research interest and is especially
interested in improving the quality of research done at all levels in Emergency
Medicine.
Pitfalls in Focused Assessment with Sonography for Trauma
FAST has become a routine part of the
initial assessment of adult patients with blunt abdominal trauma. The evidence
base for FAST is discussed and it will be shown that the vast majority of the
published literature is of low methodological quality. Studies with better
methods have found lower accuracy with FAST for the detection of free fluid.
The mean detectable free fluid in FAST is approximately 600mL in the RUQ and
150mL in the pelvis. The pitfalls of performing FAST will be demonstrated using
real cases to show how sonographer ability, patient
and environmental factors may all contribute to false or inconclusive results
with FAST. The results of a recently updated Cochrane Systematic review on the
utility of FAST based trauma management compared to non-FAST management found
that FAST-based algorithms reduced time to definitive treatment by 98 minutes
and reduces the number of CT scans requested by 52%. Although there was no
difference in mortality, RR 1 (95% CI 0.5-2.0), the wide confidence interval
suggests that more evidence is needed to determine whether FAST based trauma
algorithms impact on mortality.
Oral Cyclo-oxygenase
2 Inhibitors for Acute Soft Tissue Injury
Question: Acute Soft Tissue Injuries,
(ASTI) are common and carry significant societal costs. Cyclo-oxygenase
2 Inhibitors (COXIB), non-selective Non-Steroidal Anti-Inflammatory Drugs (NSAID)
and other analgesics are used to treat ASTI, with ongoing debate about their
analgesic efficacy, effects on tissue healing and side effects. The aim of this
dissertation is to review the evidence for oral COXIB compared to other oral
analgesics for ASTI, using outcomes: Pain, Swelling, Function and Adverse
Events.
Methods: Randomised controlled trials were
sought through a systematic search in major databases (Medline, Embase, Cochrane CENTRAL, CInAHL,
AMED, PEDro and Sport Discus), ‘grey’ literature (Clinical
trials registries and dissertations), correspondence with pharmaceutical
companies and hand searches of relevant journals. There was no language
restriction. Potentially relevant studies were screened by two reviewers for
suitability and risk of bias. Data was extracted using a standard form and
extrapolated into suitable format for analysis. Where appropriate, results were
pooled in meta-analysis. The evidence was graded for quality.
Findings: COXIB are equal to NSAID (Day 7+,
n=1884, 100mmVAS), WMD = 0.18mm (95%CI -1.76 to 2.13)) and Tramadol
(Day7+, n=706, 100mmVAS) WMD = -6.6mm (95%CI -9.63 to -3.47) for treating pain
after STI (differences not clinically significant). COXIB have less
gastrointestinal adverse effects than NSAID, even with short term use, RR =
0.59 (95%CI 0.41 to 0.85), I2 =7% (low quality evidence). COXIB are unlikely to
be different to NSAID in helping patients return to full function, however they
may improve time to return to function (moderate quality evidence) and may have
less side effects than Tramadol (very low quality
evidence). The risk of serious adverse reactions with both COXIB and NSAID in
this setting is low (but incompletely defined)
Implications: More studies comparing COXIB
to NSAID for analgesic efficacy in the setting of acute soft tissue injury are
not necessary. More evidence is required for the comparisons between COXIB and
other analgesics. The potential for early re-injury with COXIB and NSAID should
be the subject of future randomised controlled trials. Different review
methodology is required to answer the question of cardiovascular risk with
short term use of COXIB and NSAID.
Investigation of Shortness of Breath in the
ED & the Cost of Meeting Targets: The UK 4-hour Rule
The UK government has spent £43 Billion
modernising the NHS over the last 5 years. The results of a systematic review
of the published and grey literature will be presented to show that despite the
vast sums of money spent and strict adherence to the target of moving patients
out of the Emergency Department within 4 hours of arrival, there is very little
outcome data available to assess the effect of this investment and change in
practice. There is low quality evidence suggesting that there has been a slight
reduction (20min) in patient ED Length of Stay, with no reduction in the median
time to see a doctor. Only 40% of critically unwell patients are seen by a
senior doctor within 8 hours of admission, and there has been little or no
reduction in admission rate and mortality. Conversely, there has been a 13%
increase in number of investigations done per patient. The hidden costs of
investigating prior to clinical assessment are discussed using 2 real cases of mis-diagnosis based on inappropriate D-Dimer
tests. Appropriate use of D-dimer in a diagnostic
algorithm is discussed, introducing the concept of calculating a post-test
probability from a pre-test probability and a likelihood ratio (test accuracy
estimate) to inform patient management. The lack of specificity of bedside
markers to distinguish between different causes of Shortness of Breath will be
discussed, emphasising the need for proper clinical assessment prior to
requesting investigations for Shortness of Breath in the ED.
How to Search the Medical Literature
Drawing on my experience from a Masters in
Evidence Based Healthcare, I will discuss how to search the medical literature
in a concise and cohesive manner to maximise the utility of your searches and
how to tailor your search strategy to your educational / research objectives. The
importance of a Focused Question broken down into Population, Intervention,
Comparison, Outcome and Time will be emphasised. How to adapt this format to
different settings will be discussed, as will the differences between searching
the major medical databases and EBM summaries (ACP Journal Club, Cochrane,
Up-to-Date etc). A hierarchy of databases and other information sources will be
presented, with the Cochrane Collaboration stressed as the first port of call
for reliable summary information. The need to search databases other than
Medline (Embase, CINAHL etc) will be stressed. How to
reduce bias in your search by including the Grey literature and databases in
languages other than English in your search will be revealed. Practical tips
for searching using free-text and Boolean operators will be demonstrated. The
need to manage your search and results with software such as EndNote® will be discussed. Finally, you will be shown how
to integrate your search into your research and/or real time clinical practice.
Speaker: Ms. Maud Huiskamp, Toronto, Ontario, Canada
Maud has worked 10 years as an advanced
care paramedic in Toronto before becoming Lead Educator for Sunnybrook-Osler
Centre for Prehospital Care (SOCPC) in 2007. Her team
is responsible for the development of continuing education for 2000 paramedics
in Ontario. SOCPC is an essential part of disaster management in Ontario,
working with local ambulance services to provide medical oversight during
disaster situations. In addition Maud has worked with Centennial Colleges IDEAS
Network to develop and conduct live simulated mass casualty exercises and
tabletop exercises for all sectors including government, health, business,
education volunteers and media.
Role of prehospital
response systems in disasters.
A structured role for an Emergency Medical
System (EMS) is an integral part of the overall response to disaster
situations. The response must be fully coordinated with allied services and
based on effective communication among all responders. The primary roles of EMS
are triage of all the casualties, provide appropriate prehospital
medical treatment, establish a staging area, coordinate and track the movement
of all casualties, update hospitals, communicate appropriate destinations for
all patients with receiving facilities and EMS dispatch. All this is done while
ensuring the safety of all the EMS responders. Every responder must have
training and practice with regard to their roles and responsibilities during a
disaster situation, from the first responding paramedic, to the on-site EMS
Incident Manager. The operational system is supported through planning,
logistics and finance. Special roles may be assigned depending on the size and
cause of the disaster. In order for effective medical communication to occur,
the EMS system and the receiving facilities should use the same criteria for
triage. In Canada the Canadian Triage Acuity Score is utilized. EMS systems
have also established special destination algorithms to minimize the time to
definitive clinical interventions for specific patients during non-disaster
situations. Examples of these algorithms include: Field trauma triage
guidelines, On site medical team, Stroke bypass, and
STEMI bypass.
Speaker: Ms. Karen Bachynski, Toronto, Ontario, Canada
Karen is with the Ontario CritiCall Program. The CritiCall
Program is housed online within the Ontario Resource Registry along with
various other programs. Karen held the role of Call Specialist overseeing the
call centre and all patient aspects of the program for the first 8 yrs and
subsequently managed all disaster or “code orange” situations that occurred
provincially relating to the call centre during that time. With the real time,
24 hr, provincial “snapshot” of the emergency health care resources within the
province, CritiCall plays a key role in disasters /
code orange situations such as the Ice Storm 98, SARS 03 and Air France plane
crash 06. In 2004, Karen was appointed Toronto / GTA / Central East Regional
Project Manager and is responsible for the 35 hospitals within those areas as
well as the Provincial Disaster Liaison for CritiCall.
In 2008 the program under went significant restructuring under the Critical
Care Secretariat and became CritiCall Ontario. Karen
was appointed the Program Manager for LHIN’s 7, 8
& 9W as well as Provincial Liaison for Adult Acute Care & Disaster
Management.
CritiCall Ontario & Disaster Management
CritiCall Ontario is a one-number-to-call, 24-hour-a-day
consultation and referral service for physicians caring for critically ill patients
in the province of Ontario, Canada. At roughly four-times the size of Oman,
Ontario is 1.5 million square kilometres with a population of 12 million. Since
1996, CritiCall Ontario has been functioning as a
‘medical 9-1-1’service for physicians and using technology, including an
Internet-based Provincial Bed and Resource Registry, to bridge geographical
distance so that patients can be cared for quickly and appropriately.
The Provincial Bed/Resource Registry houses
vital information including on-call physician rosters, pager numbers, and
resources by specialty (including all beds at the local, regional or provincial
level). All of this information is available at the click of a mouse and
reportable through extensive provincial databases. During SARS, all patients
were transferred via CritiCall to maintain
epidemiological links, ensure effective use of limited specialized resources,
and provide statistical tracking.
CritiCall manages disaster situations using the very
same methodology. During all disaster events, the appropriate and methodical
transfer of patients is paramount and statistical tracking invaluable. In a
disaster situation, manual and electronic fan-out alerts are sent from CritiCall to local, regional and /or provincial agencies
including hospitals, EMS, and the Ministry of Health to confirm and create bed
availability, alert all specialists, and control non-disaster related patient
flow. In the interim, hospitals can spend their time taking care of patients. CritiCall’s ability to function effectively during a
disaster scenario is largely due to the fact that the protocols and resources
are in place and inform CritiCall’s day-to-day
activities of helping physicians care for their critically ill patients.
Speaker: Dr. Russell D. MacDonald University
of Toronto, Toronto, Ontario, Canada
Dr. Russell MacDonald is a specialist in
emergency medicine and is Canada’s first sub-specialist in emergency medical
services (EMS). He also has a Master Degree in Public Health from Boston
University. Dr. MacDonald has worked in academic teaching centers
and held academic appointments in emergency medicine in both Canada and the
United States. His expertise includes transport medicine, disaster
preparedness, and international health. Dr. MacDonald is the Medical Director
at Ornge Transport Medicine, North American’s largest
transport medicine agency. He is also an Assistant Professor in the Faculty of
Medicine and holds an appointment at the Institute of Medical Sciences at the
University of Toronto. Dr. MacDonald practices emergency medicine at Sunnybrook
Health Sciences Center in Toronto, and is the
Co-Director of the University’s Emergency Medicine Fellowship Program. His
academic interests include patient safety and adverse events, impact of airway
interventions on patient outcome, and the relationship between public health
and emergency services. Dr. MacDonald has a number of peer-reviewed
publications and grants for transport medicine research, and is on the
editorial board of a peer-reviewed journal.
First Do No Harm: Controversies in Prehospital Airway Management for Trauma Victims
There remains controversy over advance
airway management in the transport setting. Traditionally, paramedics with
advanced training and scope of practice have included tracheal intubation as
part of their routine management of trauma victims. There are recently
published prehospital studies that provide new
evidence regarding this practice. At first glance, the evidence appears
contradictory and the practice of airway management differs greatly across
transport systems. What the studies clearly demonstrate is that prehopsital intubation is harmful, particularly in patients
with traumatic brain injury. The question, however, remained whether or not it
was intubation itself or some other variables that causes this poor outcome.
Prehospital intubation is used to protect the airway,
oxygenate, ventilate, and deliver goal-directed therapy to patients. Further
examination of the evidence demonstrates that intubation may cause or exacerbate
hypoxia, and when comparing hypoxia and hypotension, hypoxia (and not
hypotension) is the predictor of mortality. The evidence also demonstrates that
hypocapnia or hypercapnia, due to hyperventilation or hypoventilation (respectively)
also contribute to worse outcomes. In other words, monitoring of oxygen
saturation end-tidal CO2 and directing care to maintaining oxygen
saturation and CO2 within predetermined parameters influenced
patient outcome. The evidence also demonstrates that provider skill level is a
major determinant of patient outcome. Success is related to access to and
experience with intubation, suggesting that not all intubators
are the same.
In summary, the evidence shows that
highly-skilled prehospital providers with appropriate
monitoring capabilities actually improve survival for trauma patients. This is
particularly true for those patients with traumatic brain injury.
References
1. Stiell IG, Nesbitt LP, Pickett W, Munkley D, Spaite DW, Banek J, et al. The OPALS Major Trauma Study: impact of advanced life-support
on survival and morbidity. CMAJ 2008;178(9):1141-52.
2. Liberman M, Mulder D, Sampalis
J. Advanced or basic life support for trauma: meta-analysis and mcritical review of the literature. J Trauma 2000;49(4):584-99.
3. Davis DP, Hoyt DB, Ochs M, Fortlage D, Holbrook T, Marshall LK, Rosen P. The effect of
paramedic rapid sequence intubation on outcome in patients with severe
traumatic brain injury. J Trauma 2003;54(3):444-53.
4. Dunford JV, Davis DP, Ochs M, Doney M, Hoyt DB.
Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid
sequence intubation. Ann Emerg Med 2003;42(6):721-8.
5. Chi JH, Knudson MM, Vassar MJ, McCarthy MC,
Shapiro MB, Mallet S, et al. Prehospital hypoxia
affects outcome in patients with traumatic brain injury: a prospective
multicenter study. J Trauma 2006;61(5):1134-41.
6. Warner KJ, Cuschieri
J, Copass MK, Jurkovich GJ,
Bulger EM. The impact of prehospital
ventilation on outcome after severe traumatic brain injury. J Trauma 2007;62(6):1330-8.
7. Fakhry SM, Scanlon
JM, Robinson L, Askari R, Watenpaugh
RL, Fata P, Hauda WE, Trask
A. Prehospital rapid sequence intubation for head
trauma: conditions for a successful program. J Trauma 2006;60(5):991-1001.
8. Davis DP, Peay J,
Serrano JA, Buono C, Vilke
GM, Sise MJ, Kennedy F, Eastman AB, Velky T, Hoyt DB. The impact of aeromedical
response to patients with moderate to severe traumatic brain injury. Ann Emerg Med. 2005 Aug;46(2):115-22.
9. Poste JC, Davis
DP, Ochs M, Vilke GM, Castillo EM, Stern J, Hoyt DB.
Air medical transport of severely head-injured patients undergoing paramedic
rapid sequence intubation. Air Med J. 2004 Jul-Aug;23(4):36-40.
New tools and techniques to manage the
patient’s airway
Health care professionals now have a number
of devices to help manage a patient’s airway, particularly in the setting of a
difficult airway. Some are simple and straightforward, while others require
training and skill to master their use. As health care professionals, we need
to examine these new devices and determine if and how they can be incorporated
into our practice. Depending on your scope and role as a health care provider,
these four new devices, the King Airway, AirTraq, Glidescope, and Cric, may play a
role in your practice.
The King Airway is a disposable supraglottic airway. It is designed for blind insertion in
a patient who is either breathing spontaneously or requires positive pressure
ventilation. The curved tube has a ventilation aperture positioned between two
inflatable cuffs. The distal cuff seals the esophagus
and the proximal cuff seals the oropharynx. Its role
in airway management is a possible first-line airway for basic providers or
first responders, and as a rescue device for advanced
providers as part of a difficult or failed airway algorithm. Studies have
demonstrated it can be placed faster than a combitube
or tracheal tube in simulated difficult airway scenarios.
The AirTraq is an
optical laryngoscope, providing a visual guidance system for intubation. It
holds the tracheal tube and works in place of a laryngoscope. The intubator looks through the device’s eyepiece to view the
airway, identify anatomy, and insert the tracheal tube through the cords under
direct visualization. It has a role in the patient with anticipated airway
problems due to abnormal anatomy, as a rescue device for the failed airway, for
patients with cervical spine immobilization, or removal of airway foreign
bodies under direct visualization. Studies have demonstrated it allows novice intubators to acquire intubation skill faster, and shortens
time to securing the airway in difficult airway scenarios.
The GlideScope is
a video laryngoscope that works in place of a laryngoscope to provide a fiberoptic view of the airway on a separate monitor. It has
a role is similar to the AirTraq, shortens time to
learn for novice learners, and improves success for experienced intubators. The GlideScope is available
in a portable version, making it possible to use it in combat or prehospital settings, and a small neonatal version is
available for very small airway anatomy.
The Cric is a cricothyrotomy system that can help secure the airway
surgically. It is quite new and government approval is pending for this device.
The device has a built-in scalpel, skin retraction system, and guide to
introduce a cricothyrotomy tube. The goal of the device
is to improve safety when performing an open cricothyrotomy.
While no studies have been published regarding its use, it may have a role in
the difficult or failed airway algorithm where a surgical airway is required.
When considering these four, and any other,
airway device the practitioner must reflect several things. They must consider
how they typically secure an airway, what they do in the event of failure, and
what tools are available in their practice setting to manage airways. Only then
can they consider what role a new device may have in their practice.
References
1. Russi CS. Comparison of King-LT to endotracheal
intubation and Combitube in simulated difficult
airway. Prehosp Emerg Care
2008; 12(1):35-41.
2. Guyette FX. King airway use by air medical
providers. Prehosp Emerg
Care 2007; 11(4):473-6.
3. Maharaj CH. Learning and performance of tracheal intubation
by novice personnel: a comparison of the Airtraq and
Macintosh laryngoscope. Anaesthesia 2006; 61(7):671-7.
4. Nowicki TA. Comparison of use of the the Airtraq with direct laryngoscopy by paramedics in the simulated airway. Prehospital Emergency Care 2009; 13(1):75-80.
Preparation of a Patient for Interfacility Transport
Patients frequently require transfer from
hospital to hospital to receive specialized care. However, the transfer itself
takes the patient out of a secure care setting and into a potentially unstable
environment with limited resources. In order to properly prepare a patient for
and safely carry out an interfacility patient transfer
without unnecessary risk to the patient, the practitioner must carry out
several important steps. These steps typically occur in parallel.
The first step is to quickly recognize an
illness or injury that requires a higher level of care. A patient with any
illness or injury that requires specialist or subspecialist care, advanced
investigations or technology, specialized therapy, or any service not available
locally will need to be transferred. The provider should be aware of their own
scope of practice, and the resources available to them locally. The sooner the
provider recognizes an injury or illness that exceeds their scope or local
resources, the sooner they can initiate the transfer. This is particularly
important if the illness or injury required treatment that is time-sensitive.
The second step is to
resuscitate and stabilize the patient to the best of their abilities. The
includes a proper primary survey to identify and address immediate threats to
life, carry out secondary survey to ensure no injuries are missed, and provide
adequate patient monitoring until the transfer takes place. Investigations
should never delay transfer. The common pitfalls in this step include
inadequate airway protection, no gastric decompression or spinal immobilization,
inadequate vascular access or volume resuscitation, or failure to treat the
precipitating illness. If you think the patient may need an intervention or
treatment en route, initiate it prior to transfer because doing it en route
will be more difficult.
The third step is to arrange the transfer.
This requires direct communication between the staff at the sending and
receiving facilities. Direct communication facilitates exchange of patient
information and helps ensure the patient is well prepared. The sending staff
should send copies of all documents, investigations, and other pertinent
records with the patient to the receiving facility.
The fourth step is to carry out the
transfer. The actual transfer may take place using a land ambulance, a
helicopter, or an airplane. The choice of crew is as important as the choice of
vehicle because the crew must be able to continue patient care en route. All
providers should be aware of what vehicle and crew transfer resources are
available in their area, how to access them, and what level of care they can
provide. They should also know under what circumstances hospital-based staff or
a team may be required to carry out the transfer.
The final step is to review the transfer
after it is complete. Each transfer is unique and will have lessons to help
improve subsequent transfers. This continuous process will improve the quality
and safety of future transfers; ensuring patients receive the best possible
care en route.
Speaker: Prof. Francesco Della Corte
University A. Avogadro, Novara, Italy
Dr. Della Corte is a full Professor of
Emergency and Critical Care Medicine. He graduated in 1979 at the Catholic
University, School of Medicine where he became Associate Professor in Disaster
Medicine in 1992. He has been a full Professor since 2002. He was appointed as
Secretary of the European Society for Emergency Medicine in 1999 to 2006. He is
the Course Director of the European Master in Disaster Medicine hosted by the
University of Novara and Brussels. He is the Chair of the CCU and Critical ED
at Maggiore Hospital, School of Medicine in Novara.
Do simulation exercise for hospital
management increase outcome in MCIs and disasters ?
F. Della Corte, PL Ingrassia
F. La Mura Università del Piemonte
Orientale “A. Avogadro”, SCDU Anestesia e Rianimazione, Ospedale Maggiore della Carità
The Hospital Disaster Preparedness (HDP)
Course, a modular course issued within the European Master in Disaster Medicine
(EMDM), has as its main goal how to set up Hospital Preparedness for the
admission of a large number of casualties in the Hospital. One of the course’s
motivations is that almost everywhere in the world a well defined law defines
the need for a Hospital plan for the admission of a large number of patients
after a mass casualty/disaster but very often, even if a plan has been set up,
it has never been tested with a drill or in real emergencies. The main
objective of the course are the following: to identify the possible risk
factors in a given area, to manage resources that will be used to implement the
plans, to test the plans within a simulated disaster through a simulation game
provided on a proper simulation environment.
1. Identifying the possible risk factors in
a given area: welcome to Riceland ® virtual Land
The Virtual HDP geopolitical area is called Riceland ®, and every virtual citizen (actually users from the whole
world) is given a ‘passport’ and a virtual identity to get in. Riceland is a
land composed of towns, mountains, rivers, lakes, industrial infrastructures.
Every player has a key-role, such as Major, Hospital Director, Minister, etc,
and she/he is expected to interact both with the other players and with the
Game Masters. The land is provided as a digital land embedded in a web-based
e-learning environment.
2. Managing the resources that will be used to
implement the plans: spending two months in Riceland
The citizens are expected to play the game
during a time-frame of two months (in the EMDM version of HDP). This
differentiates Riceland from any other existing MCGs:
simulation of ‘real life’ in the sense that the effect of a
political, economical, logistical and medical decisions has rebounds
over time and may contribute to prevent a disaster situation, or to improve the
risk. Despite Riceland gives a graphical interactive representation of what
happens in the digital land, it has the characteristics of a role playing game,
too. At the end of the two months, the players and their teams – led to a
completely new situation compared to the initial one (and always different from
previous “matches” played on the same platform) – must upload on the e-learning
environment their own Hospital Disaster Preparedness plan. The teams have the
possibility to ask for structural changes in the buildings (e.g., one more
back-up Hospital entrance), and must face the eventuality that big
infrastructure failure happen following a disaster simulated in Riceland. The
disasters are not driven by Artificial Intelligence agents, but are
administered by the team of Masters supervising the game, and implemented and
experienced within the game platform.
3. Testing the plans within a simulated
disaster through a simulation game provided on a proper simulation environment
Once the Teams are satisfied with their
work, the HDP plans belonging to each single Hospital in Riceland (there are
5-6 main towns and hospitals currently) will be tested using a Networked
Virtual Environment for real-time disaster simulation. Every Hospital (and all
the Teams) will experience massive influx of casualties, the issues related to
the communications with the EMS during the virtual drill, the communications
(or lack of) with other Hospitals and structures, the occurrence of sudden
infrastructure failure such as building failure (even Hospital building), roads
failure, etc. Eventually, the very next day after the virtual real-time
simulation, the EMDM Students take part to a real-size drill, organized and
arranged as it was Riceland (the virtual game) the day of the disaster. During
the live-in course of the Fifth Edition of the EMDM, 30 Students from all over
the world experienced the drill organized in the town of Casalvolone
(Novara, Italy): almost the whole town was the Set of the simulation, with real
building failures (fires, etc), real policemen, firemen, Red Cross, and –
particularly – more than 50 casualties played by the undergaduate
Medical Students of the sixth year, who ‘learned’ Disaster medicine being
‘realistic’ victims, and changing their status according to good or bad moves
taken by the players.
Conclusions
Training tools alone are not likely to be
effective without an instructional framework giving them a sense. Moreover, the
tools commonly used for training in emergency medicine seems not necessarily to
be the best ones within a Disaster medicine educational setting, since they
mostly focus on post-event reduction of the level of “improvisation” within the
acute phase occurring in a narrow time-frame and a very specific area, rather
than enlarging the scope to a wider area (metropolitan, national,
international) and a wider time-frame (including the pre-disaster assessment
phases, etc). The HDP – Riceland ® experience was very encouraging both on the
teachers and the learners perspective, and fulfilling
the possible criteria for effective simulation exercises in Disaster medicine.
References
1. Delooz H, Debacker M, Della Corte
F (2003) The European Master Program in Disaster Medicine. International
Journal of Disaster Medicine, 1; 35-41
2. Della
Corte F, La Mura F, Petrino R (2005), E-learning as
educational tool in emergency and disaster medicine teaching, Minerva Anestesiol 71:181-95
Focus on severe head injury management
prior to the OR and the ICU
F. Della Corte, GL Vignazia,
F. La Mura - Università del Piemonte
Orientale “A. Avogadro”, SCDU Anestesia e Rianimazione, Ospedale Maggiore della Carità
Traumatic brain injury (TBI) represents a
relevant pathology in the ED and still remains the leading cause of death among
people younger than 25 years of age (1) and the main single factor in
determining prognosis in the polytraumatised patient.
Prognosis in head injury has been strictly correlated with the degree and
duration of ischemia and more than 90% of autopsies on patients dead after a
head injury showed ischemic lesions of different severity (2). Many causes have
been advocated for posttraumatic cerebral ischemia such as intracranial
hypertension, arterial hypotension, brain edema and
swelling, focal tissue compression from intracranial hematomas and involvement
of microcirculation and vasospasm of large cerebral arteries. Many in vivo
studies have confirmed that ischemia is the predominant cerebral blood flow
pattern in the early postraumatic phase in head
trauma patients (3). In addition, early ischemia has been found to correlate with
poor outcome and early mortality. The correlation between CBF and mortality is
lost after this early period whereas it remains valid for cerebral metabolic
rate for oxygen (4). This pattern of early ischemia cannot be attributed only
to abnormally low cerebral perfusion pressure, excessive hyperventilation or
vasospasm, because it is still present even after normalisation of the haemodynamic and respiratory parameters, suggesting the
presence of an increased distal vascular resistance due to different factors
(extrinsic microvascular compression by damaged and edematous astrocytic processes,
active muscular contraction of the resistance arterioles caused by the trauma
induced release of vasoactive substances such as
calcium, cathecolamines, prostaglandins, haemoglobin,
neuropeptides and intravascular thrombosis)(5).
The cornerstones of treatment in the ED (6)
must be aimed at the assessment of the ABC’s and the simultaneous resuscitation
in the primary survey to obtain a normal oxygenation (arterial haemoglobin
oxygen saturation > 95%), the maintenance of CO2 values at 35
mmHg (avoiding hyperventilation with resulting hypocarbia
and subsequent hypoperfusion), the rigorous
maintenance of normal systemic arterial pressure values (avoiding even short
episodes of arterial hypotension). Prehospital
hypotension, defined as a single observation of a systolic blood pressure <
90 mmHg, has been found to be an independent predictor of outcome (7), a normal
hematocrit and normovolemia,
the avoidance of hyponatriemia and hyperglicemia, the control of epileptic seizures only if
they are clinically visible.
Notwithstanding these assumptions, prompt
diagnosis and early surgical treatment of intracranial masses still remains the
central point for the management of TBI.
Potential strategies for future therapy for
the early hypoperfusion phase include induced
arterial hypertension; pharmacological agents that might reverse the increase
in small vessels’ resistance or therapies (drugs and/or hypothermia) that
protect neurones from ischemia-induced biochemical events.
As far as the pharmacological approach is
concerned, a complex interplay of multiple mechanisms is involved in the
determination of posttraumatic cerebral injury. Available evidence suggests
that the principal players are: excitatory aminoacid
neurotoxicity, intracellular calcium overload, activation of the arachidonic acid cascade, induction of free radical-induced
lipid peroxidation, opiate peptides, cathocolamines, cholinergic neurotransmitters, cytokines
and adhesion molecules
Experimental studies on animal models
brought very promising results for neuroprotection of
the injured brain but most of them failed to demonstrate consistent usefulness
when applied in human trials. Recent phase III randomised, controlled, placebo
vs. drug studies (using competitive NMDA-receptor antagonists, CPP and CGS
19755) failed to demonstrated their efficacy in
humans.
Finally, a great interest has been directed
to the discovery that primary brain injury stimulates the cells of the CNS to
produce a variety of mediators (interleukin 1b, tumour necrosis factor alfa and interleukin 6, leucocyte
adhesion molecules (ICAM)-1, E selectin, L-Selectin, P-Selectin and Integrins, which are expressed on the surface of leucocytes
and endothelial cells, control the migration of leucocytes into tissues).
References
1. White R.J., Likavek
M.J. The diagnosis and initial management of head injury. N. Eng. J. Med;
327:1507-1511,1992
2. Graham D.I., Adams J.H. Ischemic brain
damage in fatal head injuries. Lancet 1:265-266, 1971
3. Bouma G.J., Muizelaar J.P., Choi S.C., Newlon P.G., Young H.F. Cerebral circulation and metabolism
after severe traumatic brain injury: the elusive role of ischemia J. Neurosurg. 75:685-693, 1991
4. 30-Cerebral blood flow determination with a
new DSPET device: prognostic implication in the first 48 hours after severe
head injury. Della Corte F., Giordano A., Pennisi
M.A., Barelli A., Caricato
A., Campione P., Galli G. Acta Neuchirurgica, Vol 139, n. 7, 636-642, 1997
5. Martin NA, Patwardhan
RV, Alexander MJ, Afrik CZ, Lee JH, Shalmon E, Hovda DA, Becker DP
Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia and
vasospasm. J. Neurosurg. 87; 9-19, 1997
6. Bullock R., Chesnut
RM, Clifton F, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner
MJ, Wilberger JW Guidelines for the management of
severe head injury. New York: Brain Trauma Foundation, 1996
7. Chesnut RM, Marshall LF, Klauber MR et al. The role
of secondary brain injury in determining outcome from severe head injury. J
Trauma; 34:216-222, 1993
Biomarkers in early diagnosis of septic
shock
F. Della Corte, E. Turucz,
R. Vaschetto - Università
del Piemonte Orientale “A. Avogadro”, SCDU Anestesia e Rianimazione, Ospedale Maggiore della Carità
Severe sepsis is characterized by acute
organ dysfunction secondary to infection while septic shock is defined as
severe sepsis plus hypotension not reversed with fluid resuscitation 1;2.
The mortality from severe sepsis still remains unacceptably high. A
comprehensive analysis of the epidemiology of severe sepsis in the USA during
1995 reported a mortality rate of 28.6%, amounting to 215 000 deaths
nationally, estimated to be 9.3% of all the deaths in the USA that year and
equivalent to the number of deaths following acute myocardial infarction 3.
Among the reasons suggested for the high mortality rate from severe sepsis were
late recognition of the disease and inappropriate treatment prior to admission
to the intensive care unit (ICU). Thus a key role might be played by the
emergency physicians. A recent study showed that the incidence of severe sepsis
or septic shock in patients admitted to a UK teaching hospital via its
emergency department (ED) is approximately 30 cases per 1000 4. Severe
sepsis and septic shock require comprehensive, aggressive and time-dependent
resuscitation in the ED. Recent literature has demonstrated multiple novel
options for the management of severe sepsis and septic shock 5-7.
Rivers and colleagues showed that early and aggressive resuscitation of
severely septic patients in the ED resulted in a substantial improvement on
mortality 7. On the same line, appropriate and promptly
antimicrobial therapy decreases mortality in septic patients 8. As
such, specific therapeutic options should be instituted in the ED to provide a
morbidity and mortality benefit. Discrimination between systemic inflammatory
response syndrome (SIRS) and sepsis is crucial to promptly establish
appropriate treatments in critically ill patients, since therapies and outcomes
greatly vary in patients with and without infection. Early detection of sepsis,
however, is not easy and no single clinical or biological indicator has so far
won unanimous acceptance.
Many potential biomarkers have been
investigated, but only C-reactive protein (CRP) and procalcitonin
(PCT) are currently used on a routine basis 9;10.
Combining information from several markers improves diagnostic accuracy in
detecting bacterial versus nonbacterial causes of inflammation. Multiplex
immunoassay approach has been applied in a medical ED and department of
infectious diseases of a university hospital in Denmark. They showed that
measurements of soluble urokinase-type plasminogen activator (suPAR),
soluble triggering receptor expressed on myeloid cells (sTREM)-1
and macrophage migration inhibitory factor (MIF) had limited value as single
markers, whereas PCT and CRP exhibited acceptable diagnostic characteristics 11.
Shapiro and colleagues utilized a biomarker panel to predict organ dysfunction,
shock, and in-hospital mortality in ED for patients with suspected sepsis.
Although original and theoretically useful, further study is warranted to
prospectively validate the clinical utility of these biomarkers and the sepsis
score utilized in risk-stratifying patients with suspected sepsis 12.
Recently, we characterized a new potential
marker involved in sepsis called osteopontin (OPN) 13.
Serum osteopontin levels are strikingly higher in
patients with sepsis compared to those with SIRS, and decreased during the
resolution of both the disorders 13. Furthermore we showed that OPN
levels directly correlated with those of interleukin 6 and in vitro,
recombinant osteopontin increased interleukin 6
secretion by monocytes in both the absence and
presence of high doses of lipopolysaccharide 13.
In conclusion early sepsis management in
the ED is paramount for optimal patient outcomes. Multiple early markers are
studied to improve early diagnosis and management of this complex syndrome.
Reference
1. American College of Chest
Physicians/Society of Critical Care Medicine Consensus Conference: definitions
for sepsis and organ failure and guidelines for the use of innovative therapies
in sepsis. Crit Care Med. 1992; 20: 864-74
2. Dellinger RP, Carlet
JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche
J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman
JL, Vincent JL, Levy MM: Surviving Sepsis Campaign guidelines for management of
severe sepsis and septic shock. Intensive Care Med. 2004; 30: 536-55
3. Angus DC, Linde-Zwirble
WT, Lidicker J, Clermont G, Carcillo
J, Pinsky MR: Epidemiology of severe sepsis in the
United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303-10
4. Majuran M, Clancy M: Determination of the size of the different sepsis
categories presenting to a UK teaching hospital emergency department. Emerg.Med J 2008; 25: 11-4
5. Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach
JM, Capellier G, Cohen Y, Azoulay
E, Troche G, Chaumet-Riffaut P, Bellissant
E: Effect of treatment with low doses of hydrocortisone and fludrocortisone
on mortality in patients with septic shock. JAMA 2002; 288: 862-71
6. Bernard GR, Vincent JL, Laterre
PF, LaRosa SP, Dhainaut JF,
Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr.: Efficacy and safety
of recombinant human activated protein C for severe sepsis. N.Engl.J.Med.
2001; 344: 699-709
7. Rivers E, Nguyen B, Havstad
S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich
M: Early goal-directed therapy in the treatment of severe sepsis and septic
shock. N.Engl.J.Med. 2001; 345: 1368-77
8. Leone M, Bourgoin
A, Cambon S, Dubuc M, Albanese J, Martin C: Empirical antimicrobial therapy of
septic shock patients: adequacy and impact on the outcome. Crit
Care Med 2003; 31: 462-7
9. Carrigan SD, Scott G, Tabrizian M: Toward resolving
the challenges of sepsis diagnosis. Clin.Chem. 2004;
50: 1301-14
10. Meisner M: Biomarkers of sepsis: clinically useful? Curr.Opin.Crit
Care 2005; 11: 473-80
11. Kofoed K, Andersen
O, Kronborg G, Tvede M,
Petersen J, Eugen-Olsen J, Larsen
K: Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor,
soluble urokinase-type plasminogen
activator receptor, and soluble triggering receptor expressed on myeloid
cells-1 in combination to diagnose infections: a prospective study. Crit Care 2007; 11: R38
12. Shapiro NI, Trzeciak
S, Hollander JE, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson
KJ, Milzman D, Gaieski DF, Goyal M, Cairns CB, Ngo L, Rivers EP: A prospective,
multicenter derivation of a biomarker panel to assess risk of organ
dysfunction, shock, and death in emergency department patients with suspected
sepsis. Crit Care Med 2009; 37: 96-104
13. Vaschetto R, Nicola S, Olivieri C, Boggio
E, Piccolella F, Mesturini
R, Damnotti F, Colombo D, Navalesi
P, Della Corte F, Dianzani U, Chiocchetti
A: Serum levels of osteopontin are increased in SIRS
and sepsis. Intensive Care Med 2008;
ACKNOWLEDGEMENT
The Oman Medical Specialty Board and Oman
Medical Journal’s Editorial Board would like to thank Dr. Ammar
Al-Kashmiri, Senior Consultant Emergency Physician and member of the OMSB
Emergency Medicine Scientific Committee, for his efforts in collecting and the
abstracts to be published in the journal.