An Overview of Clinical Pharmacology of Ibuprofen
Rabia Bushra1 and Nousheen Aslam2
doi:10.5001/omj.2010.49
ABSTRACT
Ibuprofen was the first member of Propionic acid derivatives introduced in 1969. It is a popular domestic and over the counter analgesic and antipyretic for adults and children. Ibuprofen has been rated as the safest conventional NSAID by spontaneous adverse drug reaction reporting systems in the UK. This article summarizes the main pharmacological effects, therapeutical applications and adverse drug reactions, drug-drug interactions and food drug interactions of ibuprofen that have been reported especially during the last 10 years.
From Ziauddin College of Pharmacy, Ziauddin University, Kaarchi, Sindh, Pakistan.
Received: 21 Feb 2010
Accepted: 24 Apr 2010
Address correspondence and reprint request to: Rabia Bushra, Ziauddin College of Pharmacy, Ziauddin University, Kaarchi, Sindh, Pakistan
E-mail: rabia_pharmacist@hotmail.com
INTRODUCTION
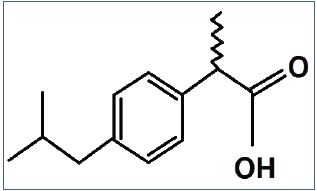
Ibuprofen is (2RS)-1[4-(2-methyl propyl) phenyl] propionic acid (BP. 2004). Ibuprofen was the first member of propionic acid derivatives to be introduced in 1969 as a better alternative to Aspirin. Gastric discomfort, nausea and vomiting, though less than aspirin or indomethacin, are still the most common side effects.1
Ibuprofen is the most commonly used and most frequently prescribed NSAID.2,3 It is a non-selective inhibitor of cyclo-oxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2).4 Although its anti inflammatory properties may be weaker than those of some other NSAIDs, it has a prominent analgesic and antipyretic role. Its effects are due to the inhibitory actions on cyclo-oxygenases, which are involved in the synthesis of prostaglandins. Prostaglandins have an important role in the production of pain, inflammation and fever.5
Clinical Pharmacology of Ibuprofen
Ibuprofen is supplied as tablets with a potency of 200 to 800 mg.6 The usual dose is 400 to 800 mg three times a day.7 It is almost insoluble in water having pKa of 5.3.8 It is well absorbed orally; peak serum concentrations are attained in 1 to 2 hours after oral administration. It is rapidly bio-transformed with a serum half life of 1.8 to 2 hours. The drug is completely eliminated in 24 hours after the last dose and eliminated through metabolism.9,10 The drug is more than 99% protein bound, extensively metabolized in the liver and little is excreted unchanged.11
Although highly bound to plasma proteins (90-99%), displacement interactions are not clinically significant, hence the dose of oral anti-cogulants and oral hypoglycemic needs not be altered.1 More than 90% of an ingested dose is excreted in the urine as metabolites or their conjugates, the major metabolites are hydroxylated and carboxylated compounds.12,6
Old age has no significant effects on the elimination of ibuprofen.13 Renal impairment also has no effect on the kinetics of the drugs, rapid elimination still occur as a consequence of metabolism.14 The administration of ibuprofen tablets either under fasting conditions or immediately before meals yield quiet similar serum concentrations-time profile. When it is administered immediately after a meal, there is a reduction in the rate of absorption but no appreciable decrease in the extent of absorption.15
Figure 1: Structural formula of ibuprofen
Therapeutic Applications
A low dose ibuprofen is as effective as aspirin and paracetamol for the indications normally treated with over the counter medications.16 It is widely used as an analgesic, an anti inflammatory and an antipyretic agent.17-19 Recemic ibuprofen and S(+) enantiomer are mainly used in the treatment of mild to moderate pain related to dysmenorrhea, headache, migraine, postoperative dental pain, management of spondylitis, osteoarthritis, rheumatoid arthritis and soft tissue disorder.20 A number of other actions of NSAIDs can also be attributed to the inhibition of prostaglandins (PGs) or thromboxane synthesis, including alteration in platelet function (PGI2 and Thromboxane), prolongation of gestation and labor (PGE2, PGF2A), gastrointestinal mucosal damage (PGI2 and PGE2), fluid and electrolyte imbalance (renal PGs), premature closure of ductus arteriosus ( PGE2) and bronchial asthma (PGs).21
The main therapeutic applications of ibuprofen are as follows:
Patent Ductus arterosus (PDA)
This is a frequent complication in premature infants. So far, intravenous indomethacin is the standard mode of medical therapy.22 However, because of adverse effects of indomethacin, other PG inhibitors such as ibuprofen have been studied for the closure of ductus arteriosus, and results indicated that ibuprofen is as effective as indomethacin.23
Rheumatoid and osteo-arthritis (RA and OA)
Ibuprofen is widely used in the management of numerous inflammatory, musculoskeletal and rheumatic disorders, because they are highly effective having minimal toxicities. 24,25 Ibuprofen 2400 mg per day resulted in rapid improvement and complete resolution of gouty arthritis within 72 hours.26 In doses of approximately 2400 mg daily, it is equivalent to 4g of aspirin in terms od anti inflammatory effects.27 Higher doses, 1200 to 1600 mg per day have been compared with a number of NSAIDs and it has been found to be as effective and well tolerated.28 Osteoarthritis is very common and treatment involves NSAIDs, particularly ibuprofen.29,30 For control of joint symptoms, diclofenac, ibuprofen, tolmetin and naproxen are equally effective.31 Roughly 1% of rheumatoid arthritis (RA) patients receiving NSAIDs are prone to develop major GI bleeds.32 With ibuprofen, gastric toxicity has been observed in 10 - 32% of patients.33
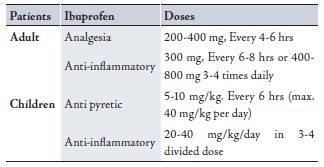
Table 1: Doses of Ibuprofen in adult & Children (34)
Cystic fibrosis (CF)
High dose ibuprofen therapy has also been shown to be effective in decreasing inflammation, probably by decreasing polymorphonuclear cell influx into the lungs.35 The risk of developing GI side effects from high dose ibuprofen therapy is low in patients with CF.36,37
Orthostatic hypotension
Ibuprofen is useful in the treatment of severe orthostatic hypotension as with other NSAIDs.38 Toxic effects are unlikely at doses below 100 mg/kg but can be life-threatening or severe above 400 mg/kg.39 However, large doses do not indicate that the clinical course is likely to be lethal.40
Dental pain
Ibuprofen is one of the most effective and widely used NSAID in treatment of dental pain.41 Dental practitioners have relied on ibuprofen and other NSAIDs to manage acute and chronic orofacial pain.42 A dose of 400 mg of ibuprofen provides effective analgesic for the control of postoperative pain after third molar surgery.43 A liquid gel preparation of ibuprofen 400mg provides faster relief and superior overall efficacy in post surgical dental pain.27
Dysmenorrhea, fever and headache
Non-prescription ibuprofen is useful for managing minor aches and pains, reducing fever and relieving symptoms of dysmenorrhea.44-46 Dysmenorrhea is the most common menstrual complain.47 Ibuprofen was superior to placebo for pain relief and menstrual fluid PGF2 alpha suppression.48 Cycloxygenase inhibitors reduce the amount of menstrual prostanoids release, with concomitant reduction in uterine hyper contractility.49 Over-the-counter (OTC) ibuprofen preparations are mainly used for acute indications, such as fever or headaches, especially tension type headache.50,51,52
It has been reported that the combined use of paracetamol and ibuprofen reduce fever very rapidly.53 Fever almost invariably accompanies uncomplicated falciparum malaria. In a randomized double-‘blind’ study, a single dose of ibuprofen was compared with paracetamol for the treatment of fever >38·5 °C due to uncomplicated falciparum malaria. Ibuprofen was significantly more effective than paracetamol in lowering temperatures throughout the first 4-5 hrs after dosing and thus should be considered as an antipyretic agent in the management of uncomplicated falciparum infections, providing there is no contraindication to its use.54 Evers et al. in 2006, conducted a double blind study to investigate the efficacy of zolmitriptan and ibuprofen in the treatment of migraine in children and adolescents. Pain relief rates after two hours were 28% for placebo, 62% for zolmitriptan and 69% for ibuprofen.55
Prophylaxis of Alzheimers disease
The administration of NSAIDs, particularly ibuprofen markedly reduced neurodegeneration.56,57 In some studies, ibuprofen showed superior results compared to placebo in the prophylaxis of Alzheimer’s disease, when given in low doses over a long time. Further studies are needed to confirm the results before ibuprofen can be recommended for this indication.58
Parkinson’s disease (PD)
Inflammation and oxidative stress have been implicated as pathogenic mechanisms in PD.59 Epidemiologic evidence showed that regular use of NSAIDs, particularly non aspirin COX inhibitors such as ibuprofen lower the risk of PD.60,61 It induced apoptosis significantly in early and late stages, suggesting that these anti-inflammatory agents might inhibit microbial proliferation.62
Breast cancer
Harris et al. in 1999 conducted a study for utilization of NSAIDs in breast cancer. Breast cancer rate was decreased by approximately 50% with regular ibuprofen intake and 40% with regular aspirin intake. Results suggested that specific NSAIDs may be effective chemo preventive agents against breast cancer.63
Adverse Reactions
NSAIDs are widely used, frequently taken inappropriately and potentially dangerously.64 Nevertheless, ibuprofen exhibits few adverse effects.65 The major adverse reactions include the affects on the gastrointestinal tract (GIT), the kidney and the coagulation system.66 Based on clinical trial data, serious GIT reactions prompting withdrawal of treatment because of hematemesis, peptic ulcer,67 and severe gastric pain or vomiting showed an incidence of 1.5% with ibuprofen compared to 1% with placebo and 12.5 % with aspirin.68 Ibuprofen was a potential cause of GI bleeding,69,70 increasing the risk of gastric ulcers and damage, renal failure, epistaxis,71-74 apoptosis,75 heart failure, hyperkalaemia,76 confusion and bronchospasm.77 It has been estimated that 1 in 5 chronic users (lasting over a long period of time) of NSAIDs will develop gastric damage which can be silent.78
Other adverse effects of ibuprofen have been reported less frequently. They include thrombocytopenia, rashes, headache, dizziness, blurred vision and in few cases toxic amblyopia, fluid retention and edema. Patients who develop ocular disturbances should discontinue the use of ibuprofen.34 Effects on kidney (as with all NSAIDs) include acute renal failure, interstitial nephritis, and nephritic syndrome, but these very rarely occur.27
Drug-Drug Interactions
Ibuprofen has established drug interactions with NSAIDs which are both pharmacokinetic or pharmacodynamic in origin.79,80 The most potentially serious interactions include the use of NSAIDs with lithium, warfarin, oral hypoglycemics, high dose methotrexate, antihypertensives, angiotensin converting enzyme inhibitors, b-blockers, and diuretics. Anticipation and care full monitoring can often prevent serious events when these drugs are used concomitantly.81
Observational studies and in-vivo experiments have raised concerns that the cardio protective effects of taking aspirin are blocked by ibuprofen which competitively inhibits aspirin’s binding sites on platelets.82-85 The pharmacodynamic interactions of aspirin and ibuprofen may not have a significant impact on patient outcomes.86 Palmer et al. in 2003 suggested that NSAIDs interfere with certain antihypertensive therapies. Ibuprofen caused a significant increase in systolic and diastolic blood pressure compared to placebo.87 A case of life-threatening hypotension due to sinus arrest was described in a patient in whom exercise-induced hyperkalemia developed during a stable regimen that included verapamil, propranolol, and ibuprofen.88 Similar to other NSAIDs, ibuprofen is likely to decrease the diuretic and anti hypertensive actions of thiazides, furosemide and b-Blockers.1
Hence the administration of ibuprofen caused a significant decrease in urinary output, inulin clearance, sodium excretion, osmolar clearance, free water clearance and urinary PGE2 clearance.89
Many overdose experiences have been reported in medical literature.90 The maximum daily dose for ibuprofen is 3200 mg. Ibuprofen may cause serious toxicity when overdosed, mainly in children on ingestion of 400 mg/kg or more. The symptoms of high dose include seizures, apnea, and hypertension, as well as renal and hepatic dysfunction.91-94 Ibuprofen has been implicated in elevating the risks of myocardial infraction, particularly among those chronically using high doses.95-100
Desmopressin and NSAIDs should not be used in combination in patients with bleeding disorders.101 Coadministration of thiopurines and various NSAIDs (ketoprofen and ibuprofen) may lead to drug interactions.102
It has been observed that caffeine improves antinociceptive efficacy of some non-steroidal anti inflammatory drugs (NSAIDs) in several experimental models, however, these effects have been questioned in humans. Caffeine is able to potentiate the antinociceptive effect of ibuprofen. This effect was greater than the maximum produced by morphine in the experimental conditions.103 Caffeine also enhances the effectiveness of most analgesics, including ibuprofen. Comparison of the cumulative response scores revealed a trend toward a greater response to ibuprofen-caffeine treatment of headaches.104
Gemfibrozil moderately increases the AUC of R-ibuprofen and prolongs its t (1/2), indicating that R-ibuprofen is partially metabolised by Cytochrome P2C8 (CYP2C8). The interconversion of R- to S-ibuprofen can explain the small effect of gemfibrozil on the t (1/2) of S-ibuprofen. However, the gemfibrozil-ibuprofen interaction is of limited clinical significance.105
St. John’s wort is a popular herbal supplement that has been involved in various herb-drug interactions. St. John’s wort treatment appears to significantly reduce the mean residence time of S-ibuprofen, no ibuprofen dose adjustments appear warranted when the drug is administered orally with St. John’s wort, due to the lack of significant changes observed in ibuprofen area under the curve (AUC) and maximum concentration C (max) for either enantiomer.106
The effects of the antifungals voriconazole and fluconazole on the pharmacokinetics of S-(+) - and R-(-)-ibuprofen were studied by Hynninen et al. A reduction of ibuprofen dosage should be considered when ibuprofen is coadministered with voriconazole or fluconazole, especially when the initial ibuprofen dose is high due to the inhibition of the cytochrome P450 2C9-mediated metabolism of S-(+)-ibuprofen.107
The competitive binding characteristics of ibuprofen and naproxen with respect to the binding site on bovine serum albumin (BSA) were studied. Ibuprofen displaced naproxen and vice versa from its high affinity binding site (site II) and the displaced drug rebound to its low affinity binding site (site I) on BSA molecule.108
Anandamide, an endocannabinoid, is degraded by the enzyme fatty acid amide hydrolase which can be inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs). The antinociceptive interaction between anandamide and ibuprofen was synergistic. The combination of anandamide with ibuprofen produced synergistic antinociceptive effects involving both cannabinoid CB1 and CB2 receptors.109
A study by Kaminski et al. in 1998 showed that all NSAIDs enhanced the protective activity of valproate magnesium against maximal electroshock-induced seizures. Only ibuprofen and piroxicam enhanced the anticonvulsive activity of diphenylhydantoin. Ibuprofen also decreased the effective dose 50 (ED50value) of valproate (for the induction of motor impairment). Thus, NSAIDs could enhance the protective activity of antiepileptics.110
Food-Drug Interaction
The absorption of ibuprofen and oxycodone when given as a combination tablet was affected by the concomitant ingestion of food. Food intake before the administration of a single dose of the combination did not affect ibuprofen absorption but marginally increased the extent, but not the rate, of oxycodone absorption.111 The effect of food on the plasma concentration-time profile of sustained release dosage forms of ibuprofen has been investigated after an overnight fast or along with a heavy vegetarian breakfast. The formulation exhibited multiple peaks on the plasma concentration-time curve. Although food did not affect the bioavailability of ibuprofen, there was a statistically significant increase in the mean concentration. Results indicated that while qualitative changes in the plasma concentration versus time curves are primarily influenced by the nature of the formulation and the type of meal, bioavailability is influenced by the absorption characteristics of the drug as well.112
The Cmax and AUC0-alpha of ibuprofen were significantly increased after a single and multiple doses of Coca-Cola, thereby indicating an increased extent of absorption of ibuprofen. The daily dosage and frequency of ibuprofen must be reduced when administered with Coca-Cola.113 Garba et al. in 2003 conducted a study indicating that Tamarindus indica fruit extract significantly increased the bioavailability of Ibuprofen.114
Warnings
The use of OTC products containing aspirin, acetaminophens, ibuprofen, naproxen or ketoprofen may increase the risk of hepato-toxicity and gastrointestinal hemorrhage in individuals who consume three or more alcoholic drinks daily.115
Tamburini et al. have reported an atypical presentation of meningitis due to Neisseria meningitidis in a patient who received large doses of ibuprofen. Anti-inflammatory therapy such as NSAIDs could reduce CSF inflammation and modify the clinical outcome in patients with bacterial meningitis. However, the use of NSAIDs is not recommended in bacterial meningitis due to a lack of studies.116
Ibuprofen may exacerbate severe asthma. With this perception, ibuprofen was administered for postoperative pain management to a 17-year-old boy with allergic rhinitis and previous severe asthma (at a time when well controlled), who then had a severe asthma exacerbation.117 Also, it has been reported that gastrointestinal tract anatomical abnormalities or dysmotility may be contraindications for therapy with high-dose ibuprofen in patients with cystic fibrosis.36
A closer look at the nonprescription analgesics revealed their potential harm when used by solid-organ transplant recipients.118 Excretion into breast milk is thought to be minimal, however it should be used with caution by women who are breast feeding.34
CONCLUSION
Ibuprofen is suitable for self medication with regards to its relatively wide spectrum of indication, good tolerance and safety.119 Overall, it has been rated as the safest conventional NSAID by the spontaneous adverse drug reaction reporting system in the United Kingdom.1
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
-
Tripathi KD. Non steroidal anti inflammatory drugs and anti pyretic analgesics. In: Essentials of medical pharmacology. 5th edn., Jaypee Brothers, New Delhi, 2003. p. 176.
-
Abrahm P, KI KD. Nitro-argenine methyl ester, a non selective inhibitor of nitric oxide synthase reduces ibuprofen-induced gastric mucosal injury in the rat. Dig Dis 2005; 50(9):1632-1640.
-
Bradbury F. How important is the role of the physician in the correct use of a drug? An observational cohort study in general practice. Int J Clin Prat 2004; (144):27-32.
-
Chavez ML, DeKorte CJ. Valdecoxib: A review. Clin Ther 2003; 25(3):817-851.
-
Wahbi AA, Hassan E, Hamdy D, Khamis E, Baray M. Pak Journal of Pharmaceutical Sciences 2005; 18(44):1.
-
Roberts LK, Morrow JD. Analgesic antipyretic and anti inflammatory agents and drugs wmplyed in treatment of gout. In: Hardman JG and Limbird LE editors. Goodman and Gillman’s the pharmacological basis of therapeutics. 10th ed., McGraw hill, New York, Chicago, 2001. p. 711.
-
Ritter JM, Lewis L, Mant TGK. Analgesics and the control of pain. In: A text book of clinical pharmacology. 4th ed., Arnold London, 1999. p. 216.
-
Herzfeld CD, Kummel R. Dissociation constant, solubilities and dissolution rate of some selective non steroidal anti inflammatory drugs. Drug Dev Ind Pharm 1983; 9(5):767-793.
-
Ross JM, DeHoratius J. Non narcotic analgesics. In: DiPalma JR and DiGregorio GJ editors. Basic pharmacology in medicine. 3rd ed., McGraw hill publishing company New York, 1990. p. 311-316.
-
Antal EJ, Wright CE, Brown BL, Albert KS, Aman LC, Levin NW. The influence of hemodialysis on the pharmacokinetic of ibuprofen and its major metabolites. J Clin Pharmacol 1986; 26(3):184-190.
-
Katzung BG, Furst DE. Non steroidal anti inflammatory drugs, disease miodifying anti rheumatic drugs, non opioid analgesics, drugs used in gout. In: Katzung BG editor. Basic and clinical pharmacology, 7th ed., Appliton and Lang Stamford, Connecticut, 1998. p.586, 1068.
-
Olive G. Analgesic/anti pyretic treatment: ibuprofen or acetaminophen? An update. Therapie 2006; 61(2);151-160.
-
Compreton EL, Glass RC, Hird ID. The pharmacokinetic of ibuprofen in elderly and young subjects. 1984 Boots research reports DT 84041.
-
Senekjian HO, Lee C, Kuo TH, Krothapalli R. An absorption and disposition of ibuprofen in hemodialysed uremic patients. Eur J Rheumatism and inflammation 1983; 6(2):155-162.
-
Physician’s desk reference. 51st ed., Published by Medical Economic Company, Inc. at Montvale, 1997. p. 1389-1391.
-
Moore M. Forty years of Ibuprofen use. Int J Clin Pract 2003(135):28-31.
-
Wood DM, Monaghal J, Streete P, Jones AL, Dargan PI. Fourty five years of ibuprofen use. 2006 Critical care, 10: R 44.
-
Nuzu K. Ibuprofen: highly potent inhibitor of prostaglandin synthesis. Biochim Biophys Acta., 1978; 529:493-494.
-
Adams SS, McCullough KF, Nicholson JS. The pharmacological properties of ibuprofen, an anti inflammatory, analgesic and anti pyretic agent. Arch Int Pharmacodyn. Ther 1969; 178(1):115-129.
-
Pottast H, Dressman JB, Junginger HE, Midha KK, Oestr H, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: ibuprofen. J Pharm. Sci., 2005; 94(10):2122.
-
Bhattacharya SK, Sen P, Ray A. Central nervous system. In: Das PK editor. Pharmacology, 2nd ed., Elsevier, New Delhi, 2003. p. 268.
-
Sharma PK, Garg SK, Narang A. Pharmacokinetics of oral ibuprofen in premature infants. J Clin Pharmacol., 2003; 43(9):968-973.
-
Kravs DM, Pharm JT. Neonatal therapy. In: Koda-Kimble MA, Young LV, Kradjan WA, Guglielmo BJ, Alldredge BK and Corelli RL editors. Applied therapeutics: the clinical use of drugs, 8th ed., Lipponcott William and Wilkins A Wolters Kluwer company Philadelphia New York, 2005. p. 94-23.
-
Tan SC, Patel BK, Jakson SH, Swift CG, Hutt AJ. Ibuprofen stereochemistry: double-the-trouble.Enantiomer., 1999; 4(3-4):195-203.
-
Russell TM, Young LY. Arthritic disorders: gout and hyperurecemia. In: Koda-Kimble MA, Young LV, Kradjan WA, Guglielmo BJ, Alldredge BK and Corelli RL editors. Applied therapeutics: the clinical use of drugs. 8th ed., Lipponcott William and Wilkins A Wolters Kluwer company Philadelphia New York, 2005. p. 42-45.
-
Frank WA, Brown MM. Ibuprofen in acute poly articular gout. Arthritis Rheum. Arthritis Rheum., 1976; 19(2):269.
-
Wagner W, Khanna P, Furst DE. Non-steroidal-anti inflammatory drugs, disease modifying anti rheumatic drugs, non opioid analgesics and drugs used in Gout. In: Katzung BG editor. Basic and clinical pharmacology 9th ed., McGraw hill Booston, 2004. p. 585.
-
Gall EP, Caperton EM, McComb JE. Clinical comparison of ibuprofen, fenoprofen, sodium naproxen and tolmetin sodium in rheumatoid arthritis. Journal of rheumatology 1982; 9(3):402-407.
-
Winstanley P, Walley T. Drug for arthritis. In: Medical pharmacology: a clinical core text for integrated curriculum with self assessment. Churchill Livingstone, Adinburgh, 2002. p. 105-107.
-
Calrk WG, Brater DC, Jhonson AR. Non steroidal anti inflammatory, anti pyretic analgesics. In: Goth’s medical pharmacology. 13th ed., Mosby year book. St: Louis Baltimore Booston. 1992.
-
Hollingworth P. The use of non steroidal anti inflammatory drugs in pediatric rheumatic diseases. Br. J Rheumatol., 1993; 32(1):73-77.
-
Nuki G, Ralston SH, Luqmani R. Diseases of connective tissues, joints and bones. In: Haslett C, Chilvers ER, Hunter JAA and Boon NA editors. Davidson’s principles and practice of medicine, 18th ed., Chirchil Livingstone UK, 1999. p. 842-843.
-
Coussement W. Gastrointestinal toxicology: toxicological pathology and sources of intestinal toxicity. In: Niesink RJM, DeVries J and Hollinger MA editors. Toxicology: principles and applications CRC Press, Boca Raton, New York, 1996. p. 655.
-
Burke A, Smyth E, FitzGerald GA. Analgesic-anti pyretic and anti inflammatory Agents; Pharmacotherapy of gout. In: Bruntom LL, Lazo JS and Parker KL (editors). Goodmans and Gilman’s the pharmacological basis of therapeutics. 11th ed., McGraw Hill, New York, 2006. p. 676-700.
-
Konston MW, Krenicky JE, Finney MR, Kirchner HL, Hillard KA, Hillard GB, et al. The journal of pharmacology and experimental therapeutics, 2003; 306: 1086-1091.
-
Mackey J, Anbar RD. High dose ibuprofen therapy associated with esophageal ulceration after pneumonectomy in a patient with cystic fibrosis: a case report. BMC Pediatr. 2004; 4:19.
-
Rifai N, Sakamoto M, Law T, Galpchian V, Harris N, Colin AA. Use of a rapid HPLC assay for determination of pharmacokinetic parameters of ibuprofen in paients with cystic fibrosis. Clin Chem 1996; 42(11):1812-1816.
-
Zawada E. Renal consequences of non steroidal anti inflammatory drugs. Postgrad. Med., 1982; 71(5):223-230.
-
Volans G, Hartley V, McCrea S, Monagham J. Non opioid analgesic poisoning. Clinical medicine, Clinical medicine 2003; 3(2):119-123.
-
Seifert SA, Bronstein AC, McGuire T. Massive ibuprofen ingestion with survival. J Toxicol Clin Toxicol 2000; 38(1):55-57.
-
Bhushan R, Martens J. Dissolution of enantiomers of ibuprofen by liquid chromatography: a review. Biomed. Chromatogr 1998; 12(6):309-316.
-
Moore TA, Hersh EV. Celecoxib and refecoxib. The role of Cox-II inhibitors in dental practice. J AM Dent Assoc 2011; 132(4):451-456.
-
Jones K, Seymour RA, Hawkesford JE. Synergistic interactions between the dual serotonergic, noradrenergic reuptake inhibitor duloxetine and the non-steroidal anti-inflammatory drug ibuprofen in inflammatory pain in rodents. British Journal of Oral and Maxilofacial surgey 1997; 35(3):173-176.
-
Grimes DA, Hubacher D, Lopez LM, Schulz KF. Non steroidal anti inflammatory drugs for heavy bleeding or apin associated with intra uterine- device use. Cochrane database Syst Rev., 2006; 18(4).
-
Pouresmail Z, Ibrahimzadeh R. Effects of acupressure and ibuprofen on the severity of primary Dysmenorrhea. J Tradit Chin Med 2002; 22(3):205-210.
-
Aycock DG. Ibuprofen: a monograph. Am. Pharm., NS 1991; 31(1):46-49.
-
Milsom I, Minic N, Dawood MY, Akin MD, Sapann J, Niland NF, Squire RA. Comparison of the efficacy and safety of non prescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clin. Ther 2002; 24(9):1384-1400.
-
Dawood MY, Khan-Dawood FS. Clinical efficacy and differential inhibition of menstrual fluid priostaglandin F2 alpha in a randomized, double- blind cross over treatment with placebo, acetaminophen and ibuprofen in primary dysmenorrheal. Am J Obstet Gyaneacol 2007; 196(1):35.
-
Dawood MY. Primary Dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 2006; 108(2):428-41.
-
Karttunen P, Sanno V, Paronen P, Peura P, Vidgren M. Pharmacokinetic of ibuprofen in man: a single-dose comparison of two over the counter, 200mg preparations. Int J Clin Pharmacol Ther Toxicol 1990; 28(6):251-255.
-
Diamond S, Freitag FG. The use of ibuprofen plus caffeine to treat tension-type headache. Curr. Pain Headache Rep 2001; 5(5):472-478.
-
Schoenen J. Treatment of tension headache. Rev. Neurol (Paris) 2000; 156(4):487-492.
-
Erlewyn-lajeunesse MD, Coppens K, Hunt LP, Chinnick PJ, Davies P, Higgimson IM and Banjer JR. Randomized controlled trial of combined paracetamol and ibuprofen for fever. Arch Dis Child 2006; 91(5):414-416.
-
Krishna S, Pukrittayakamee S, Supanaranond W, Ter Kuile F, Ruprah M, Sura T, et al. Fever in uncomplicated Plasmodium falciparum malaria: randomized double-‘blind’ comparison of ibuprofen and paracetamol treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1995; 89(5):507-509.
-
Evers S, Rahmann A, Kraeme C, Kurlemann G, Debus O, Husstedt IW, et al. Treatment of childhood migraine attacks with oral zolmitriptan and ibuprofen. Neurology, 2006; 67(3):497-499.
-
Melton LN, Keith AB, Davis S, Oakley AE, Edwardson JA, Morris CM. Chronic glial activation, neurodegeneration and APP immunoreactive deposits following acute administration of double- stranded RNA. Glia 2003; 44(1):1-12.
-
Casper D, Yaparpalvi U, Rempel N, Werner P. Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neurosci Lett 2000; 289(3):201-204.
-
Townsend K, Pratic AD. Novel therapeutic opportunities for Alzheimer’s disease: focus on non steroidal anti inflammatory drugs. FASEB J., 2005; 19(12):1592-601.
-
Ton TG, Heckbert SR, Longstreth WT Jr, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T and Checkoway H. Non steroidal anti inflammatory drugs and risks of Parkinson’s disease. Mov Disord. 2006; 21(7): 964-969.
-
Chen H, Jacobs E, Schwarzschild M, McCullough M, Calle E, Thun M, et al. Non steroidal anti inflammatory drug use and risks of Parkinson’s disease. Ann Neurol., 2005; 58(6):963-967.
-
Carrasco E, Casper D, Werner P. Dopaminergic neurotoxicity by 6-OHDA and MPP+: differential requirement for neuronal cycloxygenase activity. J. Nuerosci Res 2005; 81(1):121-131.
-
Elsisi NS, Darling-Reed S, Lee EY, Oriaku ET, Soliman KF. Ibuprofen and apigenin induced apoptosis and cell cycle arrest inactivated microglia. Neurosci Lett., 2005; 375(2):91-96.
-
Harris RE, Kasbari S, Farar WB. Prospective study of non steroidal anti inflammatory drugs and breast cancer. Oncol Rep 1999; 6(1):71-73.
-
Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over the counter pain relivers: focus on non steroidal anti inflammatory drugs: J Rheumatol 2005; 32(11):2218-2224.
-
Bateman DN. Time to re-evaluate gut toxicity. Lancet 1994; 343(8905):1051-1052.
-
Rocca GD, Chiarandini P and Pietropaoli P. Analgesia in PACU: non steroidal anti inflammatory drugs. Curr. Drug targets 2005; 6(7):781-787.
-
Tsokos M and Schmoldt A. Contribution of non steroidal anti inflammatory drugs to death associated with peptic ulcer disease:a prospective toxicological analysis of autopsy blood samples. Arch Pathol gLab Med 2001; 125 (12):1572-1574.
-
Dollery C. Therapeutic drugs 2nd ed., vol. 1, Churchill Livingstone Edinbugh London, 1999. p. 12.
-
Wolfe MM, Lichenstein DR, Signh G. Gastrointestinal toxicity of non steroidal anti inflammatory drugs. M. Engl.J.Med 1999; 340:1888(24)-1899.
-
Oermann CM, Sockrider MM, Konstan MW. The use of anti inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest 1999; 115(4):1053-1058.
-
Gambero A, Becker TL, Zago AS, De-Oliveir AF, Pedrazzoli J. Comparative study of anti inflammatory and ulceragenic activities of different cyclo oxygenase inhibitors. Inflammopharmacology 2005; 13(5-6):441-454.
-
Fulcher EM, Soto CD, Fulcher RM. Medications for disorders of the musculoskeletal system. In: Principles and applications. A work text for allied health professionals. Saunders, an imprint of Elsevier Science Philadelphia, 2003. p. 510.
-
Kennedy MJ. Inflammation and cystic fibrosis pulmonary disease Pharmacotherapy 2001; 21(5):5913-603.
-
Kovesi TA, Swartz R, McDonalds N. Transient renal failure due to simultaneous ibuprofen and aminoglycoside therapy in children with cystic fibrosis.N. Engl. J. Med 1998; 338(1):65-66.
-
Durkin E, Moran AP, Hanson PJ. Apoptosis induction in gastric mucous cells in vitro: lesser potency of Helicobacter pylori than E coli lipo polysaccharides, but positive interaction with ibuprofen. J. Endotoxin Res 2006; 12(1):47-56.
-
Vale JA and Meredith TJ. Acute poisoning due to non steroidal anti inflammatory drugs. Clinical features and management. Med Toxicol 1986; 1(1):12-31.
-
Rossi S (2004). Australian medicine hand book ISBN 0-9578521-4-2.
-
Rang HP, Dale MM, Ritter JM. Anti-inflammatory and immunosuppressant drugs. In: Pharmacology. 5th ed., Churchil Livingstone Edinburgh London, 1999. p. 248.
-
Pepper GA. Non steroidal anti inflammatory drugs; New perspectives on a familiar drug class. Rheumatology 2000; 35(1):223-244.
-
Garnett WR. Clinical implications of drug interactions with coxibs. Pharmacotherapy 2001; 21(10):1223-1232.
-
Hansen KE and Elliott ME (2005). Osteoarthritis. In:Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG and Posey LM editors. Pharmacotherapy; A pathophysiologic approach, 6th ed., McGraw Hill New York, pp1696-1698.
-
Gladding PA, Webster MWI, Farrell HB, Zeng ISL, Park R and Ruijne N. Antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am J Cardiol. 2008; 101(7):1060-1063.
-
Gaziano JM and Gibsom CM (2006). Potential for drug drug interaction in patients taking analgesic for mild to moderate pain and low- dose aspirin for cardioprotection. The Am J Cardiol 2006; 97(9):23-29.
-
FitzGerald GA (2003). Parsing an enigma: pharmacodynamic of aspirin resistance. Lancet, 361:542-544.
-
McDonal TM and Wei L (2003). Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 361:573-574.
-
Curtis JP, Wang Y, Portnay EL Masoudi FA, Havranek EP and Krumhols HM (2003). Aspirin, ibuprofen and mortality after myocardial infraction: reterospective cohort study. BMJ 327:1322-1323.
-
Palmer R, Weiss R, Zusman RM, Haig A, Flavin S and McDonald B (2003). Effects of nabumetone, celecoxib and ibuprofen on blood pressure control in hypertensive patients on angiotensin converting enzyme inhibitors. Am. J. Hypertense., 16(2):135-139.
-
Lee TH, Salomon DR, Rayment CM and Antman EM (1986). Hypotension and sinus arrest with exercise-induced hyperkalaemia and combined verapamil/propranolol therapy.The Am. J. Med., 80(6):1203-1204.
-
Ackerman Z, Cominelli S and Rynolds TB (2002). Effects of mesoprostol on ibuprofen- induced renal dysfunction in patients with decompunsated cirrhosis: results of double-blind placebo-controlled parallel group studies The American Journal of Gastrointenterology, 97(8):2033-2039.
-
McElwee NE, Veltri JC, Bradford DC and Rollins DE (1990). A prospective, population- based study of acute ibuprofen over dose: complication are rare and routine serum level not warranted. Ann. Emerg. Med 19(6):657-662.
-
Hall AH; Smolinske SC; Conrad FL; Wruk KM; Kulig KW; Dwelle TL; Rumack BH. Ibuprofen overdose. Ann Emerg Med. 1986; 15(11):1308-13 (ISSN: 0196-0644).
-
Marciniak KE; Thomas IH; Brogan TV; Roberts JS; Czaja A; Mazor SS
Massive ibuprofen overdose requiring extracorporeal membrane oxygenation for cardiovascular support. Pediatr Crit Care Med. 2007; 8(2):180-182 (ISSN: 1529-7535). -
McElwee NE, Veltri JC, Bradford DC, Rollins DE. A prospective, population-based study of acute ibuprofen overdose: complications are rare and routine serum levels not warranted. Ann Emerg Med. Jun 1990;19(6):657-62.
-
Vale JA, Meredith TJ. Acute poisoning due to non-steroidal anti-inflammatory drugs. Clinical features and management. Med Toxicol. Jan-Feb 1986; 1(1):12-31.
-
Hippisley-Cox J and Coupl C and. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis BMJ 2005; 330(7504):1366.
-
Bhatt DL. NSAIDS and the risk of myocardial infarction: do they help or harm? European heart journal, 2006; 27(14):1635-1636.
-
Ibuprofen Oral: AHFS detailed Monograph.
-
Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. May 27 2002; 162(10):1099-1104.
-
Almond C. Nonsteroidal anti-inflammatory agents. In: Emergency Medicine: A Comprehensive Study Guide. 4th ed. New York, NY: McGraw-Hill; 1995:792-795.
-
Garcia EB, Ruitenberg A, Madrestsma GS and Hintzen RQ (2003). Hyponatremic coma induced by desmopressin and ibuprofen in awomen with von willebrand’s disease Hemophilia, 9(2):232-234.
-
Oselin K, Anier K (2007). Inhibition of human thiopurine S-methyltransferase by various nonsteroidal anti-inflammatory drugs in vitro: a mechanism for possible drug interactions. Drug Metab Dispos 35(9):1452-1454.
-
López JRM, Domínguez-Ramírez AM, Cook HJ, Bravo Díaz-Reval MI, Déciga-Campos M and López-Muño FJ. Enhancement of antinociception by co-administration of ibuprofen and caffeine in arthritic rats Eur. J Pharmacology 2006; 544(1-3):31-38.
-
Dooley JM, Gordon KE, Wood EP, Brna PM, MacSween J, Fraser A. Caffeine as an adjuvant to ibuprofen in treating childhood headaches. Pediatr Neurol 2007; 37(1):42-46.
-
Tornio A, Niemi M, Neuvonen PJ, Backman JT. Stereoselective interaction between the CYP2C8 inhibitor gemfibrozil and racemic ibuprofen.Eur J Clin Pharmacol 2007; 63(5):463-469.
-
Bell EC, Ravis WR, Lloyd KB, Stokes TJ. Effects of St. John’s wort supplementation on ibuprofen pharmacokinetics. Ann Pharmacother. 2007; 41(2):229-234.
-
Hynninen VV, Olkkola KT, Leino K, Lundgren S, Neuvonen PJ, Rane A, Valtonen M, Vyyryläinen H, Laine K. Effects of the antifungals voriconazole and fluconazole on the pharmacokinetics of s-(+)- and R-(-)-Ibuprofen Antimicrob Agents Chemother. 2006; 50(6):1967-1972.
-
Rahman MM, Rahman MH, Rahman NN. Competitive binding of ibuprofen and naproxen to bovine serum albumin: modified form of drug-drug displacement interaction at the binding site. Pak J Pharm Sci. 2005; 18(1):43-47.
-
Guindon J, DeLéan A and Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain 2006; 121(1-2):85-93.
-
KamiNski R, Kozicka M, Parada-Turska J, Dziki M, Kleinrok Z, Turski WA and Czuczwar SJ. Effect of non-steroidal anti-inflammatory drugs on the anticonvulsive activity of valproate and diphenylhydantoin against maximal electroshock-induced seizures in mice. Pharmacological Research 1998; 37(5):375-381.
-
Kapil R, Nolting A, Roy P, Fiske W, Benedek I, Abramowitz W. Pharmacokinetic properties of combination oxycodone plus racemic ibuprofen: two randomized, open-label, crossover studies in healthy adult volunteers Clin Ther 2004; 26(12):2015-2025.
-
Pargal A, Kelkar MG, Nayak PJ. The effect of food on the bioavailability of ibuprofen and flurbiprofen from sustained release formulations. Biopharm Drug Dispos 1996; 17(6):511-519.
-
Kondal A, Garg SK. Influence of acidic beverage (Coca-Cola) on pharmacokinetics of ibuprofen in healthy rabbits Indian J Exp Biol 2003; 41(11):1322-1324.
-
Garba M, Yakasai IA, Bakare MT, Munir HY. Effect of Tamarindus indica. L on the bioavailability of ibuprofen in healthy human volunteers.Eur J Drug Metab Pharmacokinet. 2003; 28(3):179-184.
-
Corelli R (2004). Therapeutic and toxicity potential of over- the-counter agents. In: Katzung BG editor. Basic and clinical pharmacology 9th ed. McGraw Hill Boston, pp.1068.
-
Tamburini J, Grimaldi D, Bricaire F, Bossi P. Acute bacterial meningitis in a patient receiving ibuprofen. J Infect 2005; 51(4):336-337.
-
Palmer GM. A teenager with severe asthma exacerbation following ibuprofen Anaesth Intensive Care 2005; 33(2):261-265.
-
Gabardi S, Luu L. Nonprescription analgesics and their use in solid-organ transplantation: a review. Prog Transplant, 2004; 14(3):182-190.
-
Maceskova B. Use of over-the-counter drugs containing ibuprofen in self-medication. Cesk Slov. Farm 2005; 50(3):131-134.
How to cite this article
Bushra R, Aslam N. An Overview of Clinical Pharmacology of Ibuprofen. OMJ 2010 July; 25(3):155-161.
How to cite this URL
Bushra R, Aslam N. An Overview of Clinical Pharmacology of Ibuprofen. OMJ 2010 [Online] July; 25(3):155-161. Available at http://www.omjournal.org/ReviewArticle/FullText/201007/FT_AnOverviewofClinical.html