Seyed Behnam E-Din Jameie,1 Mohammad Abdolrahmani,2 Maliheh Nobakht3
doi:10.5001/omj.2010.53
ABSTRACT:
Objectives: This study examines the effects of total light deprivation on the developing lateral geniculate nucleus, the primary integration centre for visual information
Methods: Sprague-Dawley rats were reared for one month in a dark room from 7th postnatal day before eye opening. A group of rats was taken back into normal condition for 15 days, and then perfused. Coronal sections of LGN were prepared and stained with Cresyl Violet and Cytochrome Oxidase to investigate the number of neurons, volume and length, as well as neuronal activity level.
Results: The results showed that LD for one month causes progressive loss of neurons and decreases neuronal activity level in the LGN.
Conclusion: It can be concluded that during early postnatal development of the rats’ visual system, light deprivation causes structural and functional changes in LGN.
From 1Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran; 2Frontier Bioscience School, Osaka University, Osaka, Japan; 3Department of Anatomical Sciences, Iran University of Medical Sciences, Tehran, Iran
Received: 24 Jan 2010
Accepted: 07 Apr 2010
Address correspondence and reprint request to: Dr. Seyed Behnam E-Din Jameie, Cellular and Molecular Research Center, Iran University of Medical Sciences, Hammat Exp-way, Tehran, Iran.
E-mail: melika2@iums.ac.ir
INTRODUCTION
Development of the visual system (VS) is a very complex process which begins in prenatal stages and continues after birth. Visual system development after birth is caused by an increase in neuronal size and in glial cell number, sprouting, production of synapses and myelination. These changes are dependent on specific visual stimulus, light.1 Therefore, any kind of light deprivation can disturb the developing visual system.2
The most plastic stages in the development of Visual System are called Critical Sensitive Periods in which light stimulus is critical for the development of VS.3 Raising animals in total darkness (Dark Rearing) or closure of one or two eyes during critical periods (Monocular or Binocular Visual Deprivation) may change the receptive fields’ characteristics of neurons.4
Prolonged visual deprivation causes visual cortex neurons to lose their responsiveness to visual information coming from Lateral Geniculate Nucleus (LGN).
In 2002, Sengpiel and colleagues showed that retinal activity is important for the first formation of VS and it refines VS connections physiologically and anatomically.5 Studies by Nucci et al. have shown that visual deprivation affects LGN more than other parts of the VS.6 It is thought that any turbulences in input for instance light deprivation would cause dramatic changes in the structure and function of visual areas.7
This study examines the effects of total light deprivation on the developing lateral geniculate nucleus, the primary integration centre for visual information.
METHODS
In this study, Dark rearing method of light deprivation was used and the effects of LD on LGN were studied. 48 male Sparague-Dawly rats were divided into 4 groups and consisting of 12 rats each. Two of the four groups (control groups) were reared in normal conditions, 12 hours of light and 12 hours of darkness. The other two groups were taken as the trial groups, and were reared into a dark room for a month from the 7th postnatal day (PN). Group 1 (G1) (Trial 37pn): were reared in a dark room for a month; while group 2 (G2) (Control) comprised of control rats 37pn; G3 rats (Trial 52pn) were reared into a dark room for a month then taken back to normal condition for 15 days; and G4 consisted of 52 PN control rats.
The absolute darkness of the dark room was confirmed by a film analogue. The present study was carried out in Iran University of Medical Sciences, Tehran, Iran in the fall of 2007-2008. Experiments were carried out according to the European Communities Council Directive of 24 November 1986 for the care and use of laboratory animals, and the study was approved by the Animal Experiments and Ethics Committee of Iran University of Medical Sciences. All efforts were made to reduce animal suffering. The rats were initially transcardially perfused under deep ether anesthesia with a small amount of saline then with a fixative solution containing 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2. Their brains were subsequently removed from the skulls, postfixed for 18-24 hours, and processed for light and flouresent microscopy. The anatomical location of the LGN was determined, and the boundaries of the LGN according to Paxinos rat brain atlas are: laterally, the third ventricle, medially, the interthalamic tract, and rostrocaudally from interaural 5.70 mm to interaural 3.70 mm. According to Paxinos rat brain atlas, 50μm coronal sections of LGN were cut and stained.8
Two different staining methods including Cresyl Violet (to study the neuronal density, volume and length of LGN), and Cytochrome Oxidase (to study neuronal activity level of LGN) were used. The number of neurons was determined using the Olysiabioreports. All neurons were counted in every other section, and the lengths of single LGN neurons (35-45μm) were determined by the Olysiabioreports. Neural density was measured according to this formula7:
Multiplication of the total area of LGN section into the thickness of the section results in the volume of each LGN section where Nn: total number of neurons in each LGN section, V: total volume of each LGN section, A: total area of each LGN section, Tt: tissue thickness, Ns: total number of sections in each LGN, V= A*Tt, neuronal density of each LGN section = (Nn/V), neuronal density of LGN= S (Nn/V)/Ns. Thickness of the sections (50μm) was multiplied by the total number of LGN sections to count the length of LGN (length=Tt*Ns).
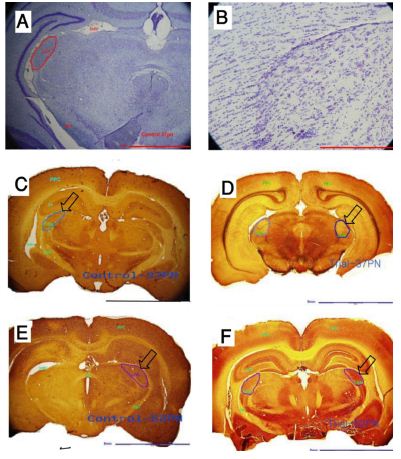
Cytochrome Oxidase (CO) stains the more active parts of the VS dark brown and the less active parts pale yellowish brown (Figs. 1E, F, G, H). CO shows the function of an area not the function of a single neuron.9 Results were analyzed using SPSS and Excel. Statistical analysis was performed using the Paired T-Test and Kruskal-wallis test.
RESULTS
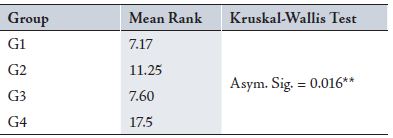
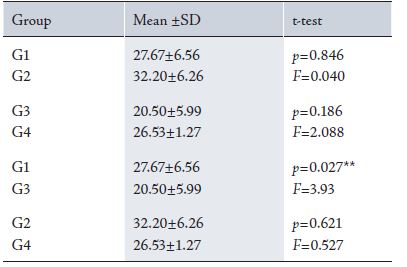
After measuring the morphometric, functional and histological parameters results showed that between G1 (Trial 37pn) and G2 (Control 37pn), results from the cresyl violet test showed that neuronal density, volume and length of the LGN were lower in G1 than in G2. (Tables 1-3, Fig 2)
Neuronal loss in G1 was not significant but the decrease in volume and length of LGN in G1 was statistically significant. While, results from the Cytochrome Oxidase test, showed that neuronal activity level of the LGN in G1 was lower than in G2. (Figs. 1C & D)
A comparison between G3 (Trial 52pn) and G4 (Control 52pn) is demonstrated in Tables 1-3, and Fig. 2. Results from the cresyl
violet test showed that neuronal density, volume and length of
the LGN in G3 were lower than those in G4, but the differences were not statistically significant. Hence, the results of Cytochrome Oxidase test showed that neuronal activity level of the LGN in G3 was lower than in G4. (Fig. 1E & F)
Table 1: Showing the mean rank of the Volume of LGN in different groups
Table 2: Showing the mean differences of length of LGN in different groups
Table 3: Showing the mean differences of Neuronal Density of LGN between different groups
Results from the cresyl violet test showed that neuronal density of the LGN in G2 (Control 37pn) was higher compared to G4
Figure 1: Cresyl Violet and Cytochrome oxidase staining of LGN. A & B: Cresyl Violet showing the density of neurons in one LGN coronal section. C (G2), D (G1), E (G4) & F (G3): Cytochrome Oxidase (CO) showing the activity level of LGN (CO stains the more active parts of visual system into dark brown and less active parts to pale yellowish brown). Arrows are indicating the LGN. Scale bars (A: 2mm, B: 500µ, C: 5mm, D: 5mm, E: 5mm, F:5 mm)
While a comparison between G1 (Trial 37pn) and G3 (Trial 52pn) is depicted in Tables 1-3 and Fig. 2.
DISCUSSION
The visual system is plastic in response to altered visual stimuli, and visual deprivation in early postnatal life causes vast changes in visual dominance and results in a decline in visual responsive neurons in the visual cortex.10 The effects of total light deprivation
including decreases in synaptic density, synaptic spines, neuronal size and number has been reported in different biological models such as primates,11 rats,12 and mice.13 Since early 20th century, different methods of visual deprivation such as enucleation,
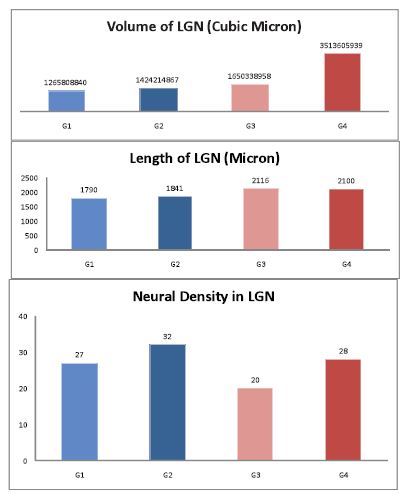
Figure 2: Histograms showing different parameters considered in this study. (G1: animals reared in dark room for a month from 7th PN; G2: control 37 PN rats; G3: reared in dark room for a month then taken back into normal condition for 15 days; and G4: control 52 PN rats.)
Monocular and binocular suture have been used to study the complex visual system and the extent and variety of changes after deprivation depend on timing and method in spite of the fact that these changes could be affected by surgical stresses.14,15,16
Since the presence of retina and its activity is critical to induce changes in the visual system, one cannot study the effects of light as a special visual stimulus by enucleation or lid suture. Among different methods, total light deprivation which was used in this study is the most appropriate method to study the effects of light. Neonate rats without any surgical operation of the retina or on any other visual areas were placed in a dark room and once the deprivation was over, they were prepared to be studied.
The results from this study showed that total light deprivation caused histological, morphometric and functional changes in LGN. The changes include neuronal loss, decrease in volume and length of LGN and decline in neuronal activity levels. (Figs. 1 & 2, Tables 1-4)
A variety of studies have shown similar results. Fifkova et al. reported that monocular lid suture in 14pn rats decreases the volume of LGN.17 While Heumann et al. in 1980, claimed that binocular enucleation causes a 27% decrease in neuronal number, 25% decrease in Glial cells number and 57% decrease in total volume of LGN.16 From this current study, it would appear that neuronal loss and decrease in volume and length of LGN was due to cell death.18
However, Nucci et al. reported that short term visual deprivation in sensitive periods causes the visual cortex to lose responsive neurons.6 Then again, Nucci et al. in 2000 showed that visual deprivation causes apoptosis in LGN of neonate rats. As a result of cell death, the number of viable neurons is progressively decreased as the deprivation period increases.18 They reported that apoptosis is responsible for neuronal loss in LGN. After deprivation retinal neurons are active enough to induce synaptic changes in LGN.19 Presynaptic retinal activity is necessary to induce cell death in LGN.18 Omitting presynaptic retinal activity by intra ocular injection of tetrodotoxin prevents increasing apoptotic cell number.20 Hence, different authors have shown that the ablation of optic nerve prevents cell death in LGN.21
The importance of retinal activity to induce deprivation mediated cell death in LGN has been confirmed further by a variety of methods that block synaptic transmission of neurotransmitters.22 In this study, the retina was intact but deprived from light, therefore, retinal activity could have been able to induce cell death in LGN. Data have shown that during visual deprivation, retinal synaptic terminals transmit a signal which could be responsible for excessive release of nitric oxide (NO) and activation of the mechanisms of apoptosis.23
The most important candidate for these changes could be glutamate, an excitatory neurotransmitter which is released by the optic nerve in LGN.23 Visual deprivation during critical sensitive periods causes abnormal stimulation of glutamate receptors in LGN which activate NO synthase (NOS). NOS activation in turn causes excessive release of NO in LGN. NO breaks the DNA which activates PARP (poly ADP-ribose polymerase, which is attributed to causing a rapid drop in energy stores of neurons which consequently leads to cell death.24 This study showed that the decrease in LGN neuronal number because of cell death is associated with a decline in neuronal activity level.24,25 This would have been achieved because the toxic activity of NO and activation of PARP inhibit mitochondrial activity in LGN and total energy of the LGN is therefore decreased.26
The decrease in neuronal density in G3 (Trial 52pn) showed that even taking the rats back into normal condition would not improve the changes caused by light deprivation. This highlights that the constant effect of light deprivation is critical during sensitive periods. Cell death occurred in LGN causing a constant decrease in neuronal density which was accompanied by decreases in volume and length of LGN in G3 (Trial 52pn). Carlson et al. in 1987 observed that short term binocular light deprivation caused deep constant changes in neuronal number and neuronal activity of the visual system in primates.27
While Nucci et al. reported that cell death causes an irreversible decrease in neuronal number of LGN, but the changes are reversible if visual deprivation ends when critical sensitive period starts.6 Results of CO in G3 (Trial 52pn) showed lower neuronal activity of LGN compared to G4 (Control 52pn). This indicates that Ad Libitum for 15 days increased neuronal activity of the remaining neurons in LGN. However, increased neuronal activity level could have also been caused by compensatory mechanisms. The recovery of LGN neuronal activity showed capabilities that the remaining neurons could possibly compensate apoptotically lost neurons.6 In addition, it has been reported that increased synaptic density after LD and Light exposure causes the neuronal activity of LGN to be increased.28,29
CONCLUSION
Overall, it could be concluded that total light deprivation during critical periods would result in the loss of neurons and neuronal activity in visual areas specifically LGN which directly receives input from both retinas.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
-
Upledger J. A brain is born, Exploring the birth and development of the Central Nervous System. 1st ed. California, North Atlantic Books, 1996.
-
Van Camp N, Verhoye M, De Zeeuw C. I, Van der Linden A. Light Stimulus Frequency Dependence of Activity in the Rat Visual System as Studied With High-Resolution BOLD fMRI, J Neurophysiol 2006; 95 (5): 3164–3170.
-
Keigo K, Akinori SM, Ito TH, Tanaka K, Hayakawa T, et al. Apoptosis and retinal projections in the dorsal lateral geniculate nucleus after monocular deprivation during the later phase of the critical period in the rat, Anat Sci Int. 2003; 78(2): 104-10.
-
Spillman S.D. 2005. First research on developmental amblyopia due to early deprivation_Hans Berger’s experiments in 1900. Perception 34(7): 765–767.
-
Sengpiel F., Kind PC. 2002. The role of activity in development of the visual system; Curr Biol. 10;12(23): 818-26.
-
Nucci C, Piccirilli S, Nistico R, Cerulli L, Bagetta G. Excitotoxic Mechanisms of Apoptosis in the Mammalian Visual System Following Monocular Visual Deprivation; Pharmacology & Toxicology 2002; 91 (4): 153–157.
-
Abdolrahmani M, Jameie SB. 2009. Gradual Increase in neuronal density of rats` lateral geniculate nucleus from anterior to posterior. Neurosciences 14 (2):124-127
-
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Amsterdam: Academic Press, 2007. p. 37-68.
-
Wong-Riley M. T.T. 1989. Cytochrom oxidase: an endogenous methabolic marker for neuronal activity. TINS. 12(3): 94- 101.
-
Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol. Rev 1982; 62 (2): 738-755.
-
Hubel DH, Wiesel T. Plasticity of ocular dominance columns in monkey striate cortex. Trans. R. Soc. London B. Biol. Sci 1997; 278 (961): 131-163.
-
Fagiolini M, Pizzorusso T, Berardi N. Functional postnatal development of the rat primary visual cortex and the role of visual experience, dark rearing and monocular deprivation. Vision Res 1994; 34 (6): 709-720.
-
Gordon JA, Stryker MP. Experience dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci 1996; 16 (10): 3274-3286.
-
Bruner JS. The Cognitive Consequences of Early Sensory Deprivation, Psychocomatic Medicine, 1959; 21( 2): 238-246.
-
Fifkova E. Changes in the Visual Cortex of Rats after Unilateral Deprivation; Nature 1968; 220 (5165): 379 – 381.
-
Heumann D, Rabinowicz T. Postnatal development of the dorsal lateral geniculate nucleus in the normal and enucleated albino mouse. Exp Brain Res. 1980;38(1):75-85.
-
Fifkova E. The effect visual and light deprivation on the retina, Experimental Neurology 1972; 35(3): 458-469.
-
Nucci C, Piccirilli S, Nistico R, Grandinetti M, Cerulli L, Leist M, et al. Apoptosis in the dorsal lateral geniculate nucleus after monocular deprivation involves glutamate signaling, NO production, and PARP activation. Biochem Biophys Res Commun. 2000; 19;278(2):360-7.
-
Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J. Neurobiol 1999; 41 (1), 83-91.
-
Nucci C, Morrone L, Rombola L, Nistico R, Piccirilli S, Cerulli L. Intra vitreal injection of tetanus neurotoxin prevents monocular deprivation induced apoptosis in the lateral geniculate nucleus of new born rats. Br. J. Pharmacol 2002; 135: 352-368.
-
Robaey MS, Clarke DB, Wang YC. 1994. Effects of ocular injury and administration of BDNF on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1994; 91(5):1632-6. 22.
-
Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80 (2): 717-766.
-
Sillito AM, Murphy PC, Salt TE, Moody CI. Dependence of retinogeniculate transmission in cat on NMDA receptors. J. Neurophysiol 1990; 63 (2): 347-355.
-
Nucci C, Morrone L, Rombola L, Nistico R, Piccirilli S, Cerulli L. Multifaceted role of nitric oxide in the lateral geniculate nucleus: from visual signal transduction to neuronal apoptosis. Toxicology Letters 2003; 139 (2-3): 163-173.
-
Dawson VL, Dawson TM. Nitric oxide in Neuronal degeneration. Proc Soc Exp Biol Med. 1996; 211(1):33-40.
-
Pieper AA, Verma A, Zhang J, Snyder SH. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999; 20 (4): 171-181.
-
Carlson S, Pertovaara A, Tanila H. Late effects of early binocular visual deprivation on the function of Brodmann’s area 7 of monkeys (Macacaarctoides). Brain Res, 1987; 430(1): 101-11.
-
Albanese A, Albanese E, Brusco A, Saavedra JP. A quantitative study of visual cortex synapses during the postnatal development of dark-reared rats; Journal of Neurobiol. 1983; 14(1): 1 – 8.
-
Gabbott P.L.A., Stewart M.G. 1987. Quantitative morphological effects of dark-rearing and light exposure on the synaptic connectivity of layer 4 in the rat visual cortex (area 17); Exp Brain Res 1987; 68(1):103-114.
How to cite this article
E-Din Jameie SB, Abdolrahmani M, Nobakht M. Effects of Total Light Deprivation on Dorsal Lateral Geniculate Nucleus of Male Neonate Rats. OMJ 2010 July; 25(3):179-183.
How to cite this URL
E-Din Jameie SB, Abdolrahmani M, Nobakht M. Effects of Total Light Deprivation on Dorsal Lateral Geniculate Nucleus of Male Neonate Rats. OMJ [Online] 2010 July; 25(3):179-183. Available at http://www.omjournal.org/OriginalArticles/FullText/201007/FT_EffectsofTotalLight.html.