Association Between Seminal Plasma Copper and Magnesium Levels with Oxidative Stress in Iraqi Infertile Men
Omar F. Abdul-Rasheed
doi:10.5001/omj.2010.51
ABSTRACT
Objectives: To study the association between copper, magnesium and malondialdehyde levels in seminal plasma of oligozoospermic, azoospermic in relation to normozoospermic men.
Methods: The present study was conducted at the Chemistry and Biochemistry department, College of Medicine, Al-Nahrain University, Baghdad-Iraq during September 2007 to February 2008 after obtaining approval from the research and ethics committee and obtaining written consent, 78 infertile men (age range 33.01±4.20 years) were recruited at the institute of embryo research and infertility treatment, Al-Kadhimiya teaching hospital, Iraq and were categorized according to their seminal fluid parameters to oligozoospermia (n=43) and azoospermia (n=35). 41 fertile men (age range 30.29±2.30 years) were selected as controls. Seminal plasma copper and magnesium were measured by atomic absorption spectrophotometry. Malondialdehyde was measured calorimetrically using thiobarbituric acid assay which detects thiobarbituric acid reactive substances.
Results: Seminal plasma copper level was decreased significantly (p=0.000) in the azoospermic group compared to the control group. Whereas, the level decreased non-significantly in the oligozoospermic group. Seminal plasma magnesium levels were decreased significantly (p=0.000) in all the infertility groups studied. On the other hand, malondialdehyde levels which is an end product of lipid peroxidation were significantly elevated (p=0.000) in all the infertility groups studied.
Conclusion: Copper and magnesium work in different ways in order to maintain normal environment for spermatozoa for normal fertilization to occur.
From the Department of Chemistry and Biochemistry, College of Medicine/ Al-Nahrain University, Al-Kadhimiya- PO. Box: 70027, Baghdad- Iraq.
Received: 11Mar 2010
Accepted: 12 May 2010
Address correspondence and reprint request to: Dr. Omar F. Abdul-Rasheed, PhD, Department of Chemistry and Biochemistry, College of Medicine/ Al-Nahrain University, Al-Kadhimiya- PO. Box: 70027, Baghdad- Iraq.
E-mail: omar_rasheed39@yahoo.com; dr.omar_rasheed@yahoo.com
INTRODUCTION
Infertility is a common clinical problem, leading approximately one in six couples in the UK to seek professional advice.1 It can be primary (conception has never occurred) or secondary, and due to problems affecting either the male or the female.1
Male infertility is defined as the inability to conceive after one year of unprotected sexual intercourse.2 Thus it continues to be a major problem for clinicians.3
The secretion and composition of testis and adnexal glands are being studied to further understand the male reproductive system.3 Inorganic elements present in the male reproductive system have secured the attention of many investigators.3
Copper is an essential trace element. It is required in the diet because it is the metal cofactor for a variety of enzymes (amine oxidase, copper – dependent superoxide dismutase, cytochrome oxidase and tyrosinase).4 Copper accepts and donates electrons and is involved in reactions involving dismutation, hydroxylation and oxygenation.4 However, excess copper can cause problems because it can oxidize proteins and lipids, bind to nucleic acids and enhance the production of free radicals.4 It is thus important to have mechanisms that will maintain the amount of copper in the body within normal limits.4 Copper after ingested in diet is carried to the liver bound to albumin, then is taken up by liver cells, and part of it is excreted in the bile. Copper also leaves the liver attached to ceruloplasmin, which is synthesized in that organ.4
The level of ceruloplasmin may be increased due to an acute-phase response, oestrogens or pregnancy.5 The normal serum copper is 10 -22 µmol/L.4 Magnesium is the fourth most abundant cation in the body. The adult human body contains approximately 1000 mmol, with about half in bone and the remainder distributed equally between muscles and other soft tissues.6 Only 11-17 mmol is found in the ECF, the plasma concentration being 0.8-1.2 mmol/L. The normal daily intake of magnesium (10-12 mmol) is greater than is necessary to maintain magnesium balance (approximately 8 mmol/ 24 hrs) and the excess is excreted through the kidneys.6 Magnesium acts as a cofactor for some 300 enzymes; including enzymes involved in protein synthesis, glycolysis and the transmembrane transport of ions.6 A magnesium-ATP complex is the substrate for many ATP-requiring enzymes such as alkaline phosphatase, Hexokinase, fructokinase, phosphofructokinase, adenyl cyclase, cAMP dependent kinases, amongst others.6-8
Magnesium is important in the maintenance of the structure of ribosomes, nucleic acids and some proteins.6 There are two major roles for magnesium in biological systems: (i) it can compete with calcium for binding sites on proteins and membranes, and (ii) it can form chelates with important intracellular anionic ligands, notably adenosine triphosphate (ATP).7,8 The normal serum magnesium level is 1.8-2.2 mg/dL. Out of this 60% is ionized, 10% is complexed with other ions and 30% is bound to proteins.8
Several powerful oxidants are produced during the course of metabolism, in both blood cells and most other cells of the body; these include superoxide (O2.-), hydrogen peroxide (H2O2), peroxyl radicals (ROO.) and hydroxyl radical (OH.) and are referred to as reactive oxygen species (ROS). Free radicals are atoms or groups of atoms that have an unpaired electron.4 Chemical compounds and reactions capable of generating potential toxic oxygen species can be referred to as pro-oxidants.4 On the other hand, compounds and reactions disposing of these species, scavenging them, suppressing their formation, or opposing their actions are antioxidants and include compounds such as nicotinamide adenine dinucleotide (NADPH), glutathione, ascorbic acid and vitamin E. In a normal cell, there is an appropriate pro-oxidant: antioxidant balance.
However, this balance can be shifted toward the pro-oxidants when the production of oxygen species is greatly increased (eg, following ingestion of certain chemicals or drugs) or when levels of antioxidants are diminished (e.g. by inactivation of enzymes involved in the disposal of oxygen species and by conditions that cause low levels of the antioxidants mentioned above). This state is called “oxidative stress” and can result in serious cell damage if the stress is massive or prolonged. ROS are now thought to play an important role in many types of cell injury, some of which can result in cell death.4 ROS are free radicals that play a significant role in many of the sperm physiological processes such as capacitation, hyperactivation and sperm-oocyte fusion. However, they also trigger many pathological processes in the male reproductive system, and these processes have been implicated in cancer of the bladder and prostate as well as in male infertility.9-12 Spermatozoa are sensitive to oxidative stress because they lack cytoplasmic defenses.12-14
Moreover, the sperm plasma membrane contains lipids in the form of polyunsaturated fatty acids, which are vulnerable to attack by ROS. ROS, in the presence of polyunsaturated fatty acids, triggers a chain of chemical reactions called lipid peroxidation.15-17 Malondialdehyde (MDA), an end product of polyunsaturated fatty acid oxygenation, is a reliable and commonly used biomarker for assessing lipid peroxidation.18 The measurement of MDA is based on its reaction with thiobarbituric acid (TBA) to form a colored MDA-TBA adduct.18 The aim of this study is to find out the relation between oxidative stress and levels of copper and magnesium in the seminal fluid from patients with different types of infertility.
METHODS
A case-control study was conducted in the Chemistry and Biochemistry department, College of Medicine/Al-Nahrain University, Baghdad, Iraq. After obtaining the approval of the research and ethics committee of Al-Nahrain Medical College and written consent from the patients, 78 infertile patients aged 33.01±4.20 years were enrolled throughout this study in the period between September 2007 and February 2008.
The patients were without any treatment and had regular unprotected intercourse for at least 12 months without conception with their partners. The wives of the infertile subjects included had no obvious causes for infertility like tubal blockage or ovulation disorders. Patients who had infertility secondary to infection, were taking medication, or had a congenital defect and had more than 106 leukocyte/mL in their semen analysis were excluded from this study. Also, individuals having diabetes or thyroid diseases, patients who were on antipsychotic or antihypertensive drugs, or taking alcohol, nicotine, vitamins, minerals and antioxidant supplementation within the past three months were also excluded from the study. 41 healthy donors with proven fertility and had initiated a successful pregnancy within the last year and had a normal spermiogram at the time of study were selected as controls.
The patients were categorized according to their seminal fluid analysis parameters to oligozoospermic (n=43) and azoospermic (n=35). Semen samples were collected from the males undergoing infertility screening at Al-Kadhmiya teaching hospital and institute of embryo research and infertility treatment/ Baghdad- Iraq. The specimens were collected in sterile plastic containers by masturbation after an abstinence period of 48-72 hours and were analyzed within one hour of collection. After allowing the specimen liquefy for 30 minutes, seminal fluid analysis was performed to measure sperm concentration, normal sperm morphology, progressive sperm motility in accordance with the recommendations of the World Health Organization (WHO).19 The WHO criteria for sperm normality used were as follows: sperm concentration ≥20 millions/mL of ejaculate, percentage of sperm progressive motility (a+b) ≥50% and normal sperm morphology ≥30%. Seminal plasma was separated by centrifugation at 800 x g for 15 minutes at room temperature. The supernatant was removed immediately and kept in 20ºC.
Seminal plasma copper and magnesium were measured by atomic absorption spectrophotometry. Seminal plasma samples were diluted (1:10) with deionized water for copper measurements and (1:50) with 1% lanthanum chloride for magnesium measurement. Copper and magnesium were measured at the wavelengths 324.7 nm and 285.2 nm respectively with Shimadzu AA-6200 atomic absorption flame emission spectrophotometer. All glassware used in the measurement of copper and magnesium were boiled in 6 mole/L nitric acid for 60 minutes and then washed twice with deionized water.
The amount of malondialdehyde (MDA) was determined by the thiobarbituric acid (TBA) assay.20-23 In brief, 100 µ of seminal plasma was diluted with deionized water to 1ml. To each diluted sample, one-half ml of thiobarbituric acid (0.67%) was added. All tubes were heated in a boiling water bath for exactly one hour and centrifuged for 10 minutes at 1000 x g, then the supernatant was separated carefully and the absorbance of the pink color formed was measured at 534 nm against an appropriate blank.
Most of the data were expressed in mean ± SD, and analyzed by one way analysis of variance (ANOVA) for multiple comparisons, and Pearson’s Correlation coefficient (r) and student t-test for paired results with significance level fixed at p=0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences computer program (SPSS for windows, version 17.0).
RESULTS
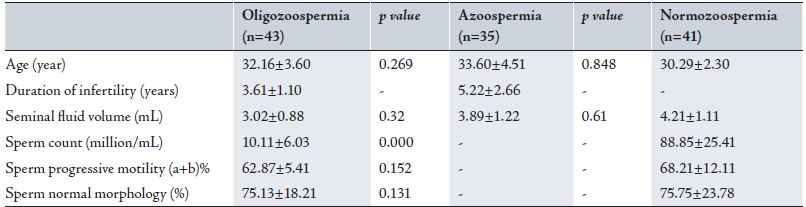
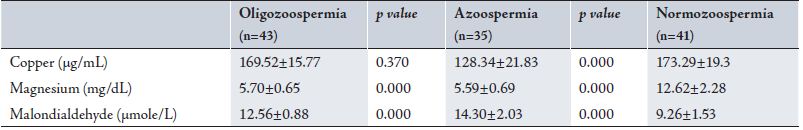
The characteristics of the subjects who participated in this study with their seminal fluid parameters are listed in Table 1. Table 2 illustrates the seminal plasma copper, magnesium and malondialdehyde levels in the oligozoosperia, azoospermia and normozoospermia groups.
Table 1: Characteristics of Patients and Sperm parameters
Table 2: Mean Seminal Plasma Copper, Magnesium and Malondialdehyde levels
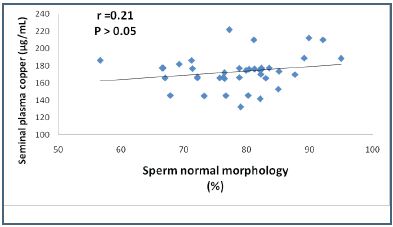
Figure 1: Pearson’s Correlation Plot of Seminal Plasma Copper levels versus Normal Sperm Morphology in control group
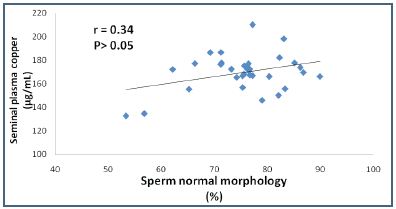
Figure 2: Pearson’s orrelation plot of seminal plasma copper levels versus normal sperm morphology in patients with oligozoospermia
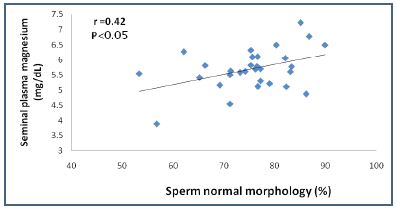
Figure 3: Pearson’s correlation plot of seminal plasma magnesium levels versus normal sperm morphology in oligozoospermic infertility group
DISCUSSION
Copper is involved in oxidation- reduction reactions and has a dominant role in diverse proteins such as cytochrome oxidase and cytoplasmic superoxide dismutase. In this study, there was a significant decrease in seminal plasma copper levels in azoospermic patients (p= 0.000) and this decrease may lead to a concomitant decrease in the superoxide dismutase activity. Therefore, the decrease in seminal plasma copper levels may be considered as an important mechanism of increased oxidative stress in the azoospermic infertile semen. On the other hand, copper reduces the oxidative processes and glucose consumption, which reduces or abolishes sperm motility.24 Generally, the trace elements zinc and magnesium found in seminal plasma originates primarily from the prostate gland and may reflect prostatic secretory function.25,26 Furthermore, such analysis is of great help in the assessment of androgen activity and the functional status of the genital glands. Infections of the prostate gland markedly decrease the concentration of zinc and magnesium in semen.27 Abou-shakra et al. reported that magnesium may play a role in spermatogenesis particularly in sperm motility.28,29
The results from this study showed that there was a very significant decrease in the infertile seminal plasma magnesium levels of oligozoospermic patients (p=0.000) and azoospermic patients (p=0.000) and this may indicate that magnesium may be a good criterion for prostate function and for good sperm quality. Also, seminal plasma magnesium correlated significantly and positively with sperm morphology (r=0.42; p<0.05) of the oligozoospermic group compared to the control group.
Levels of seminal plasma magnesium listed in Table 2 are in agreement with Deger and Akkus (1988) who reported seminal fluid magnesium levels equal to 11.9±3.0 mg/dL in fertile subjects (n=37) and 6.9±2.1 mg/dL in different forms of infertile subjects (n=45).30 On the other hand, the results from this study are unlike those from a previous study conducted by Saaranen et al. (1987).31 Hsieh et al. (2006) reported that the seminal plasma level of MDA in a control group (n=20) was 1.52±0.75 nmole/mL and 2.25±0.88 nmole/mL in the oligoasthenospermia group (n=31).22 On the other hand, Zarghami et al. (2005) mentioned that seminal plasma MDA levels were higher in the asthenozoospermic subjects rather than in the control subjects (0.72±0.06 μM versus 0.40±0.06 μM).21
The seminal plasma MDA level measured in this study was 9.26 ±1.53 μM in control group, 12.56±0.88 μM in the oligozoospermia group and 14.30 ±2.03 μM in the azoospermia group. These results were different from those obtained by Zarghami et al. in 2005 and by Hsieh et al. in 2006.21,22 Oligozoospermia and azoospermia were associated with higher seminal plasma MDA activity (p=0.000). Increased MDA activity could represent the pathologic lipid peroxidation of the spermatozoa membrane and the following inhibition of sperm motility and viability.32 A positive correlation was found in this study between the seminal plasma MDA concentration and sperm concentration (r=0.206; p>0.05) in the oligozoospermic group and this finding was incompatible with the studies of Geva et al. in 1996, Fraczek et al. in 2001 and Kobayashi et al. in 1991.32-35 Also from this study, there was no significant correlation between seminal plasma MDA and sperm progressive motility (data not listed) and this was inconsistent with the study conducted by Suleiman et al. in 1996.36 The positive association (r=0.21, 0.34 ; p>0.05) between seminal plasma copper and normal sperm morphology in the control and oligozoospermic groups respectively may indicate that copper ion is essential for maximal superoxide dismutase activity which is considered as the principal antioxidant enzyme that may lead to less free radical formation during the spermatogenesis process and to increased normal sperms formed in morphology. On the other hand, magnesium was correlated significantly and positively (r=0.42, p<0.05) in the oligozoospermic group and this finding may be caused by normal and well functioning prostate gland activity.
CONCLUSION
In view of this study and the available literature, two conclusions can be drawn. Firstly, it could be that the functions performed by different elements are not the same and this could be for providing a suitable medium for the survival of spermatozoa, stimulation its actions, facilitating the entrance to the ova during fertilization or functioning as an integral part of its membrane. The second conclusion could be that there is no direct association between malondialdehyde and copper or magnesium levels.
ACKNOWLEDGEMENTS
The author reported no conflict of interest and no funding was received on this work.
-
Marshall WJ, Bangert SK. The gonads. In: Marshall WJ, Bangert SK, editors. Clinical Chemistry. 6th ed. Mosby Elsevier, 2008. p. 200.
-
Swerdloff RS, Wang C. The testis and male sexual function. In: Goldman L, Ausiello D, editors. Cecil textbook of medicine. 22nd ed. WB Saunders, 2008. p. 1472 – 1483.
-
Skandhan KP, Makada MT, Amith S. Levels of cadmium, nickel, manganese and lead levels in normal and pathological human seminal plasma. Urologia 2005; 72(4): 461-464.
-
Murray RK. Plasma proteins and immunoglobulins. In: Murray RK, Granner DK, Rodwell VW, editors. Harper’s illustrated biochemistry. 27th ed. Lange Medical Books/ McGraw-Hill, 2006. p. 596,597.
-
Crook MA. Vitamins, trace elements, and metals. In: Crook MA, editor. Clinical Chemistry & Metabolic Medicine. 7th ed. Edward Arnold (Publishers) Ltd. 2006. p. 230.
-
Marshall WJ, Bangert SK. Calcium, phosphate and magnesium. In: Marshall WJ, Bangert SK, editors. Clinical Chemistry. 6th ed. Mosby Elsevier, 2008. p. 250.
-
Milne DB. Trace elements. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. 3rd ed. WB Saunders, 1999. p.1034.
-
Vasudevan DM, Sreekumari S. Mineral metabolism. In: Vasudevan DM, Sreekumari S, editors. Textbook of biochemistry for medical students. 4th ed. Jaypee Brothers medical publishers, 2005. p.305.
-
Bankson DD, Kestin M, Rifai N. Role of free radicals in cancer and atherosclerosis. Clin Lab Med 1993; 13(2): 463-480.
-
Hietanen E, Bartsch H, Bereziat JC, Camus AM, McClinton S, Eremin O, Davidson L, Boyle P. Diet and oxidative stress in breast, colon and prostate cancer patients: a case-control study. Eur J Clin Nutr 1994; 48(8): 575-586.
-
Agarwal A, Saleh RA. Role of oxidants in male infertility: rationale, significance, and treatment. Urol Clin North Am 2002; 29(4): 817-827.
-
Agarwal A, Prabakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J. Androl. 2005; 26(6): 654-660.
-
Donnelly ET, McClure N, Lewis SE. Antioxidant supplementation in vitro. Fertil. Steril. 1999; 72(3): 484-495.
-
Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J. Androl. 2002; 23(6): 737-752.
-
Agarwal A, Hamamah S, Shekarriz M. Reactive oxygen species and fertilizing capacity of spermatozoa. Contracept. Fertil. Sex.1994: 22: 327-330.
-
Kobayashi H, Gil-Guzman E, Mahran AM, Sharma RK, Nelson DR, Thomas AG, JR, Agarwal A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001. 22(4): 568-574.
-
Zalata AA, Ahmed AH, Allamaneni SS, Comhaire FH, Agarwal A. Relationship between acrosin activity of human spermatozoa and oxidative stress. As. J. Androl. 2004; 6(4): 313-318.
-
Sheu JY, Ku HP, Tseng WC, Chen MT, Tsai LY, Huang YL. Determination of thiobarbituric acid adduct of malondialdehyde using on-line microdialysis coupled with high-performance liquid chromatography. Anal. Sci. 2003; 19(4): 621-624.
-
World Health Organization: WHO laboratory manual for the examination of human semen and sperm- cervical mucus interaction. 4th ed, Cambridge University press, 1999.
-
Dandekar SP, Nadkarni GD, Kulkarni VS, Punekar S. Lipid peroxidation and antioxidant enzymes in male infertility. J. Postgrad. Med. 2004; 48 (3): 186-189.
-
Zarghami N, Khosrowbeygi A. Seminal plasma levels of 15-F 2α- isoprostane, malondialdehyde, and total homocysteine in normozoospermic and asthenozoospermic males. Indian J. Clin. Biochem. 2005; 20 (2): 86-91.
-
Hsieh YY, Chang CC, Lin CS. Seminal malondialdehyde concentration but not glutathione peroxidase activity is negatively correlated with seminal concentration and motility. Int. J. Biol. Sci 2006; 2 (1): 23-29.
-
Rao B, Soufir JC, Martin M, David G. Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res 1989; 24 (2): 127-134.
-
Roychoudhury S, Slivková J, Bulla J, Massányi P. Copper administration alters fine parameters of spermatozoa motility in vitro. Folia. Veterinaria. 2008; 52: 64-68.
-
Eliasson R, Lindholmer C. Magnesium in human seminal plasma. Invest. Urol. 1972; 9: 286-289.
-
Abyholm T, Kofstad J, Molne K, Stray-Pedersen S. (1981) Seminal plasma fructose, zinc, magnesium and acid phosphatase in cases of male infertility. Int. J. Androl 1981; 4 (1): 75 – 81.
-
Eliasson R. Biochemical analyses of human semen in the study of the physiology and pathophysiology of the male accessory genital glands. Fertil. Steril. 1968; 19 (3):344-350.
-
Abou – shakara FR, Ward NI, Everard DM. The role of trace elements in male infertility. Fertil. Steril. 1989; 52 (2): 307-310.
-
Krassas GE, Pontikides N, Deligianni V, Miras K. A prospective controlled study of the impact hyperthyroidism on reproductive function in males. J. Clin. Endocrinol. Metabol. 2002; 87 (8): 3667 – 3671.
-
Deger O, Akkus I. Semen magnesium levels in fertile and infertile subjects. Magnesium. 1988; 7 (1):6-8.
-
Saaranen M, Suistomaa U, Kantola M, Saarikoski S, Vanha-Perttula T. Lead, magnesium, selenium and zinc in human seminal fluid: comparison with semen parameters and fertility. Human Reproduction. 1987; 2 (6): 475 – 479.
-
Verma A, Kanwar KC. Effect of vitamin E on human sperm motility and lipid peroxidation in vitro. Asian J Androl 1999; 1(3): 151- 154.
-
Fraczek M, Szkutnik D, Sanocka D, Kurpisz M. Peroxidation components of sperm lipid membranes in male infertility. Ginekologia Polska. 2001, 72 (2): 73-79.
-
Geva E, Lessing JB, Bartoov B, Zabludovsky N, Lessing JB, Lerner Geva L, Amtta A. The effect of antioxidant treatment on human spermatozoa fertilization rate in an in vitro fertilization program. Fertil. Steril. 1996; 66 (3):430-434.
-
Kobayashi T, Miyazaki T, Natori M, Nozawa S. Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991; 6 (7):987-991.
-
Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J. Androl. 1996; 17 (5): 530-537.
How to cite this article
Abdul-Rasheed OF. Association Between Seminal Plasma Copper and Magnesium Levels with Oxidative Stress in Iraqi Infertile Men. OMJ 2010 July; 25(3):168-172.
How to cite this URL
Abdul-Rasheed OF. Association Between Seminal Plasma Copper and Magnesium Levels with Oxidative Stress in Iraqi Infertile Men. OMJ [Online] 2010 July; 25(3):168-172. Available at http://www.omjournal.org/OriginalArticles/FullText/201007/FT_AssociationBetweenSeminal.html.