Survey of Pregnancy Outcome in Preterm Premature Rupture of Membranes with Amniotic Fluid Index <5 and ≥5
Fatemeh Tavassoli,1 Marzieh Ghasemi,1 Ashraf Mohamadzade,2 Jamileh Sharifian2
Tavassoli F, et al. OMJ. 25, 118-123 (2010); doi:10.5001/omj.2010.32
ABSTRACT
Objectives:Preterm premature rupture of membranes (PPROM) is among the most important causes of perinatal morbidity and mortality. The aim of this study is to survey the pregnancy outcomes in preterm premature rupture of membranes with an amniotic fluid index of <5 and >5.
Methods:This prospective cohort study was performed on 137 pregnant women complicated by preterm premature rupture of membranes (PPROM) with a gestational age of 28-34 weeks during October 2006 to October 2008. The patients were divided in two groups according to their amniotic fluid index; AFI<5 (77cases), AFI≥5 (60cases). The Chi-squared test for qualitative variables and T-student test for quantitative variables were used to analyze the results.
Results:The results showed that there was no significant difference in terms of the number of pregnancies, gestational age at rupture of membranes and birthweight between the two groups. However, the results demonstrated that the patients with AFI<5 exhibited a significantly shorter latency period (p=0.049), a higher rate of cesarean due to fetal distress (p=0.008), a lower neonatal Apgar score in the first minute (p=0.0127) and a higher rate of neonatal death during the first week (p=0.045).
Conclusion: Overall, PPROM with oligohydroamnios is associated with shorter latency, higher rate of C/S, higher rate of early neonatal death and lower neonatal Apgar.
From the 1Department of Obstetrics and Gynecology ,Women’s Health Research Center, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran, 2Department of Pediatrics, Neonatal Research Center, Imam Reza Hospital, Mashhad, University of Medical Sciences, Mashhad, Iran.
Received: 29 Dec 2009
Accepted: 12 Feb 2010
Address correspondence and reprint request to: Dr. Marzieh Ghasemi, Department of Obstetrics and Gynecology, Women’s Health Research Center, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
E-mail: ghasemim841@yahoo.com
Tavassoli F, et al. OMJ. 25, 118-123 (2010); doi:10.5001/omj.2010.32
INTRODUCTION
Preterm premature rupture of the membranes (PPROM)
occurs in 3% of pregnancies and causes around 25-30% of all preterm deliveries.
Since PPROM is associated with lower latency from membrane rupture until
delivery, it is an important cause of perinatal morbidity and mortality.1-3
During the latency period, the ascent of pathogenic microorganisms from the
lower genital area could create complications such as intrauterine infections.4-8
Also, some studies introduced PROM as a pathologic process that often occurs
following membrane inflammation and infection. Bacterial infection in
choriodecidual levels with brief amnion involvement has been observed after
PROM.
It has been demonstrated that as many as 25-30% of women with PPROM have a
higher incidence of positive amniotic fluid culture obtained by amniocentesis
even when there is no clinical doubt for chorioamnionitis.1,9
However, one of the most common complications in PPROM patients is intrauterine
infection, which can lead to chorioamnionitis, metritis after delivery and
perinatal outcome such as neonatal sepsis.1,7 Other complications are
cord compression leading to fetal distress, cord prolapse during rupture of
membranes and placental abruption.1,7 Perinatal outcomes constitute
prematurity, neonatal sepsis, respiratory distress syndrome (RDS),
intraventricular hemorrhage (IVH), risk of fetal and neonatal death.2
When PROM occurs earlier from term, there are significant risks of maternal and
perinatal morbidity and mortality, therefore the attending physicians play an
important role in the management of PPROM. They should a develop pregnancy
outcome plan, whereby a suitable decision is reached for decreasing maternal and
fetal risks.2 Most authors have proposed a strategy for the
conventional management for women with PPROM before 34 weeks gestation,
associatiated with antibiotic and corticosteroid administration.
The main benefit of the conservative management is prolonging pregnancy which
can decrease gestational age-related morbidity associated with prematurity, but
the benefit must be balanced with the risks of conservative management, such as
clinical chorioamnionitis.1-4, 9
Since amniotic fluid has certain bacteriostatic properties which protect
against potential infection, it seems that a decrease in amniotic fluid volume
may impair the pregnant women’s ability to combat such infections and cause an
increased risk of infection.3,10,11
The aim of this study is to survey pregnancy outcome in PPROM with an amniotic
fluid index of <5 and ≥5.
This prospective cohort study was performed on 137 pregnant women with a
gestational age of 26-34 weeks diagnosed with PROM. This study was conducted at
the Imam Reza hospital associated with the Mashhad University of Medical
Sciences during October 2006 - October 2008.
All the patients were categorized into two groups according to their amniotic fluid index (AFI); AFI<5 (77cases) and AFI≥5 (60cases). The patients were controlled in labor due to the nature of their AFI. After delivery, the perinatal and maternal outcomes were evaluated in both groups.
Gestational age of 26-34 weeks was considered for this study. Gestational age was
estimated by the patients’ last menstrual period (LMP). It was determined on the
basis of whether menstruation was regular or by ultrasonography detecting
gestational age of <20 weeks. An ultrasound was used for verification when the
results of the two methods were inconsistent by more than 7 days. For the
patients who did not have a sonography, gestational age was determined by a new
sonography and comparing fundal height with the date of last menstrual period.
The other inclusion criteria included normal fetus showed in previous
sonographies, and confirmed PPROM diagnosis, which was determined by sterile
speculum examination using the pooled fluid, fern test and Nitrazine paper test.
The following parameters were used to exclude patients from this study;
multiparity, maternal background disease (preeclampsia or diabetes), symptoms of
chorioamnionitis at admission, history of previous cesarean or previous surgery
of the uterine, noncephalic presentation, intrauterine growth retardation (IUGR)
and spontaneous delivery during the first 12 hours after rupture of the
membranes.
At first, the selected patients who fit the criteria were hospitalized, admitted
into labor rooms and were controlled for 12 hours in the view of emerging
contractions, bleeding or possible start of delivery using non-assuring fetal
tests and fetal heart monitoring. Vaginal examinations were not usually
performed during hospitalization, however examinations were performed using a
sterile speculum when necessary.
If any symptoms of bleeding, contraction, fetal distress were not observed after 12
hours, and the patients did not enter the active phase of delivery, they were
transferred to the obstetrics unit for expectant management.
A sonography was performed for all the patients during the first 12-24 hours to
measure the AF in four abdominal quadrants in order to determine the AFI. Then
the patients were divided into two groups according to their AFI.
For patients who were hospitalized for more than 48 hours, a sonography was again
performed, the process of calculating the AFI was repeated and a new AFI was
determined. If the AFI had changed, the patients were grouped according to the
new sonography results.
The patients received a single course of Betamethasone at admission (2 doses
Betamethasone 12 mg every 24 hours) and they received antibiotic prophylaxis
consisting of Ampicillin with Erythromycin (firstly, two days injection and then
orally for the following five days).1,7 During hospitalization, fetal
heart rate (FHR) was controlled every two hours. Moreover, daily nonstress tests
(NST) were performed for fetus with gestational age >28 weeks.
The patients were controlled for clinical symptoms of chorioamnionitis such as
fever (controlling temperature every four hours), uterine tenderness, maternal
tachycardia, fetal tachycardia, and laboratory symptoms (leukocytosis-CRP-ESR).
Clinical diagnosis of chorioamnionitis was performed according to the presence of
at least two of the following criteria; fever before delivery at temperature
greater than 38°C or 100/4°f ( measure two or more times with 1 hour intervals),
fetal tachycardia >160, uterine tenderness, positive maternal CRP, foul-smelling
vaginal secretions and foul-smelling amniotic fluid, maternal tachycardia
>120/1min, maternal leukocytosis (WBC>20000) (12-14). If symptoms suggested the
start of clinical chorioamnionitis, antibiotics were injected and if delivery
did not start, labor was then induced.
Delivery indications included cervical dilatation of 4 cm and 80% of effacement
(spontaneous start of delivery active phase), clinical chorioamnionitis,
gestational age >34 weeks, hemorrhage and fetal distress. Cesareans were
performed only on the basis of obstetric indications. Scientific latency was
defined as the period between membrane rupture reported by the patient to the
point of delivery. Applied latency was defined as the period between the time of
membrane rupture determined by the physician to the point of delivery. Maternal
characteristics during latency were collected in order to compare between the
two groups as follows; latency length, signs of clinical chorioamnionitis,
placental abruption, meconium in AF, fetal distress, prolapsed cord, and mode of
delivery.
Neonates for every gestational age and every Apgar were
transferred to the neonatal intensive care unit (NICU) for evaluation. Then
blood culture and cerebro spinal fluid (CSF) were taken from each neonate, if
needed. The neonates were hospitalized at the NICU if needed, and the rest were
transferred to the Roming in, but they were controlled for any signs and
symptoms of respiratory distress syndrome (RDS) or possible sepsis.
Fetal evaluation of neonatal morbidity in this study included intrauterine death,
early neonatal death (first week), signs of RDS, and signs of neonatal sepsis
determined by blood or CSF positive culture during the first 72 hours after
birth. The diagnosis of RDS was confirmed when neonates presented symptoms, when
radiography confirmed hyaline membrane disease (HMD), or when respiratory
failure in neonates required supported respiration for at least for 24 hours.
Also, the diagnosis of neonatal sepsis was given when clinical results suggested
infection from positive blood culture or a sample of CSF.15,16
In this study, descriptive statistics such as frequency distribution tables,
median, mean, standard deviation, maximum and minimum values were used to
describe the studied variables in both groups. Thus the Chi-square test was used
for comparing qualitative variables between the two groups and the T-test was
used for comparing the quantitative variables between the two groups, while the
Mann-Whitney or Kruskal Wallis tests were used for comparing variables which did
not have a normal statistical distribution.
A total of 137 pregnant women with a gestational age of 26-34 weeks complicated by PPROM who fit the inclusion criteria were evaluated during a period of two years.
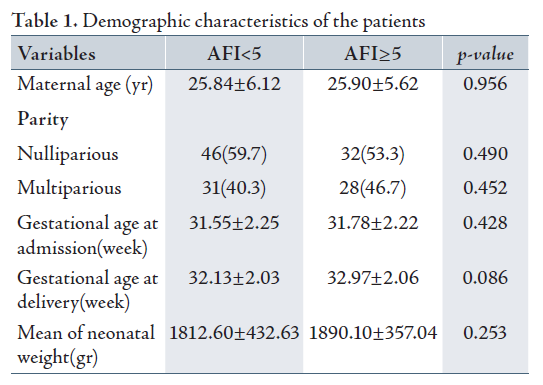
60 patients comprised group I (AFI≥5) and 77 patients comprised group II (AFI<5). The two groups represented similar maternal age at admission, parity, gestational age at delivery and birth weight since the p-value suggested that there was no significant difference between the two groups. (Table 1)
Overall, the mean gestational age was 31.64 weeks with standard deviation of 2.23 weeks at admission. While the Mean gestational age in group II (AFI<5) was 31.55 weeks and 31.78 weeks in group I (AFI≥5) according to the T-test, which showed that there was no significant difference between the two groups. The overall Mean gestational age at delivery was 32.5 weeks. Group II (AFI<5) exhibited a mean of 32.13 and group I (AFI≥5), exhibited a mean of 32.97 weeks. Hence, no significant statistical difference was observed between the two groups.
The Mean neonatal birth weight was 1846 g with standard deviation (SD) of 401.75. Group II (AFI<5) exhibited a mean birth weight of 1812.60 g and SD of 432.63, while the mean birth weight in group I (AFI≥5) was 1890.10g with a SD of 357.04. The T-test showed no significant difference between the two groups in terms of birth weight (p=0.253).
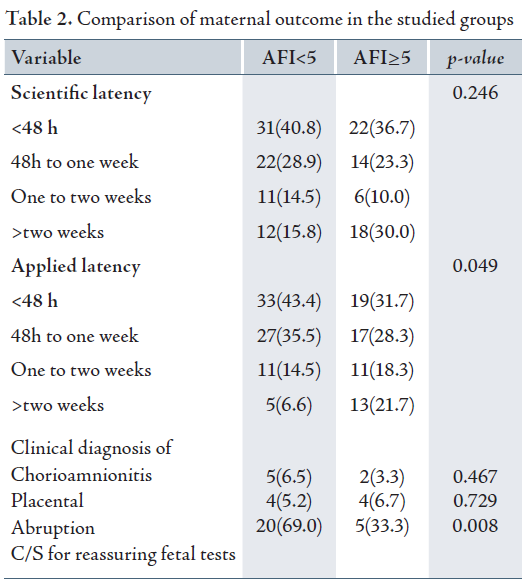
Scientific latency was not significantly different between the two groups (p=0.246), but the time of applied latency was significantly shorter in group II (AFI<5) than in group I (AFI≥5) with a p value of 0.049. Although, the signs of clinical chorioamnionitis in group II (AFI<5) was 6.5% and 3.3% in group I (AFI≥5), there was no significant difference between the two groups (p=0.467).
The two groups were similar in terms of signs of placental abruption, detachment and etiology of pregnancy termination. However, evaluation of the causes of cesarean, fetal distress were significantly higher in group II (AFI<5) compared to group I (p=0.008). (Table 2)
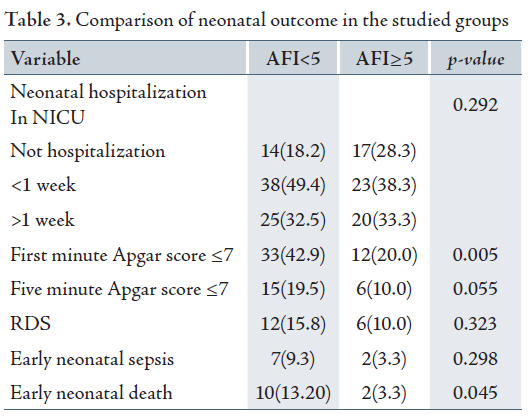
The first minute Apgar score ≤7 was significantly higher in group II (AFI<5) compared to group I (p=0.005), but although the five minute Apgar score ≤7 was higher in group II (AFI<5), there was no significant different between the two groups (p=0.055).
The mean time of neonatal hospitalization in NICU was 6.29 days with SD of 7.03 days. Hence, group II (AFI<5) exhibited a mean of 6.5 days while group I (AFI≥5) exhibited a mean of 6.02 days. Nevertheless, there was no statistical significance between the two groups (p=0.686).
Both the groups showed similar rates of respiratory distress syndrome (p=0.323) and early neonatal sepsis (p=0.298), (Table 3). The most common cause of neonatal sepsis in this study was E.coli (5 cases). Other causes included negative Staph. coagulaz (1 case), Group B Stereptococus (1 case), Entercoci klebsiela (1 case).
The overall rate of early neonatal death was 8.8%. 10 cases (13.2%) of neonatal deaths were observed in group II (AFI<5) while only 2 cases (3.3%) were recorded for group I (AFI≥5). Therefore, the rate of neonatal death was significantly higher in group II (AFI<5) compared to group I (AFI≥5) with a p value of 0.045.
DISCUSSIONPPROM causes definite maternal and neonatal morbidity and mortality; therefore, the attending physicians should be considerably aware of the risk factors and should be able to judge appropriately whether to terminate the pregnancy or to continue with the pregnancy.
Expectant management with antenatal antibiotic and corticosteroid administration are the recommended standard of care in the setting of PPROM at gestational age of ≤34 weeks.1 In terms of the bactericidal property of amniotic fluid, and its protective role against infections, it seems that a decrease in the volume of amniotic fluid after PROM increases the patient’s suscpetibility to infection, therefore, the risk of infection is increased. This hypothesis was first evaluated by Vintzileos et al. in 1985.10 They reported the relationship between oligohydroamnios (AFI<5), increased infection and perinatal mortality.
In this study, the rate of chorioamnionitis was observed at 5%, although 70% of pregnancies complicated by chorioamnionitis had AFI<5, however there was no significant difference between the two groups. This finding was in accordance with the study of Mercer et al in 2006 which showed that there was no relationship between chorioamnionitis and oligohydroamnios.12 Piazze et al. in 2007 did not find any correlation between the two groups although 66% of cases exhibited chorioamnionitis at AFI<5, however, they reported a significant relationship between higher maternal WBC and fever (temperature >38°c) with oligohydroamnios ( p 0.001).17
The results from this study were in contrast to the results of Borna et al. in 2004
and Moberg et al. in 1984, they found a significant correlation between AFI<5
and a higher rate of chorioamnionitis.18,19
In 2001, Park et al. reported a significant correlation between fluid volume and AF
positive culture.11 A Considerable point in their study was the rate
of around 5% of chorioamnionitis in PROM, whereas in some studies this rate was
reported as 13-40% and in a study by Osmanagaoglu et al. the rate was 12.2%.13,20,21
This was possibly due to the use of antibiotic prophylaxis, but also the lack of
manual examination of the patients. Overall, in the majority of previous
studies, oligohydroamnios were associated with shorter latency.
One of the advances of this study was the close time of rupture of the membranes.
At first, the definition of latency was described as the period between rupture
of the membranes until delivery that often was equal to the number of days that
the patients was hospitalized before delivery. But some patients deferred to
report the rupture of the membranes by a few days. Therefore, in order to solve
this problem, two variables were calculated; scientific latency (as the period
of rupture of the membranes until delivery according the patient’s report), and
applied latency( as the period of the time of rupture of the membranes
determined by the physician until delivery).
The Scientific latency was not significantly different between the two groups but
the applied latency was significantly different between the two groups. In group
II (AFI<5), 43% of cases delivered during the first 48 hours and only 6.6% of
pregnancies were prolonged by more than 2 weeks. However, in group I (AFI≥5),
31.7% of patients delivered during the first 48 hours and 21.7% of pregnancies
were prolonged by more than 2 weeks. Most studies have not reported this point,
but in a study by Borna et al. the latency period was observed to be equal in
both groups.20 Piazze et al. and Vermillion et al. reported
significant correlation between oligohydroamnios and latency period.17,22
Fetal distress was the most common cause of cesarean in group II (AFI<5) and
non-reaction to induction was the most common cause of cesarean in group I
(AFI≥5). There was a significant relation between cesarean due to fetal distress
and AFI<5 (p=0.008). This finding was also
consistent with the results of Borna et al. and Vermilion.22,18
The rate of cesareans in this study was 32%, whereas in a study performed in 2005,
the rate of cesarean in PROM was 21% and the rate of cesarean due to fetal
distress was 22.7%.13 Moreover, the mode of pregnancy termination in
terms of vaginal delivery or need for cesarean was evaluated in this study, but
found that there was no significant difference between the two groups in terms
of the mode of delivery. Also, in both groups, similar results were observed in
terms of placental abruption, spontaneous start of contractions and pregnancy
termination due to different causes.
Fetal death was not observed in this study, but some studies have reported 1% of
fetal death with PROM. The possible cause of the difference in results may be
due to limited number of patients and the higher gestational age in this study.
In this study, first minute Apgar score ≤7 was significantly higher in group II
(AFI<5) and five minute Apgar score ≤7 was also higher in group II (AFI<5),
however the difference was not significant between the two groups. Piazze et al.
in 2007 found that there was a significant association between five minute Apgar
score ≤7 and AFI<5 (p<0.001), but this study showed that the association was not statistically
significant. 17
Vermillion et al. reported that PPROM is associated with reduced rate of
respiratory distress syndrome.22 However, the results from this study
did not show a significant correlation between the two groups in terms of signs
of respiratory distress syndrome and PPROM. Sims et al. in 2002 reported a 17%
rate of respiratory distress in neonates with maternal PPROM.23 The
results obtained from this study were consistent with the results by Borna et
al.18
Piazze et al. motioned that AFI<5 were observed in 70% of neonates with RDS, and
this rate was reported at 66.6% in this study.17 However, this
finding was in contrast with the finding of Mercer et al. who showed that AFI<5
was associated with a higher risk of RDS (p=0.03).12
Although in this study, 77% of patients with sepsis had AFI<5, the statistical
difference was not significant between the two groups. Borna et al. reported
similar results (30%) in AFI<5 and 27/9% in AFI ≥5.18
Gonik et al. and Mercer et al. did not find any association between AFI<5 and
neonatal infections morbidity.12,24 But Vermillion et al. in 2000
reported that an AFI<5 is the only definite risk factor associated with early
neonatal sepsis (p=0.004).19 Moreover Vintzileos et al. reported the association between
oligohydroamnios and an increase of infection and perinatal mortality.10
The decreased rate of sepsis in this study may be due to the close evaluation of
patients with PROM, examining possible symptoms of clinical sepsis and early
treatment of any clinical and laboratory findings. Other causes may have been
the higher rate of the mean neonatal age (32.1 weeks) and the mean neonatal
birth weight (1840 g).
Similar studies did not report findings on neonatal death and the time of
hospitalization in NICU, therefore a direct comparison was not achievable.
PPROM with oligohydroamnios is associated with shorter latency, higher rate of C/S, higher rate of early neonatal death and lower neonatal Apgar. Therefore, it is recommended to consider the AFI as a prognosis index in patients with PROM. However, further studies with larger samples are needed to clarify the role of AFI in PROM.
The authors would like to thank the Research Faculty of Mashhad University of Medical Sciences for financially supporting this article. No conflict of interest was reported.
-
Mercer BM. Premature rupture of the membrane. In: Petraglia F, Strauss GF, Gabbe SG, Wises G. Complicated Pregnancy. 4th ed. London: informa health care; 2007. p.713-727 .
-
Weissmann-Brenner A, O’Reilly-Green C, Ferber A, Divon MY. Values of amniotic fluid index in cases of preterm premature rupture of membranes. J Perinat Med. 2009; 6. [Epub ahead of print].
-
Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child 1988; 63:1146-1150.
-
Pasquier JC, Picaud JC, Rabilloud M, Claris O, Ecochard R, Moret S, et al. Neonatal outcomes after elective delivery management of preterm premature rapture of the membranes before 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol 2009; 143:18-23.
-
Keyon SL, Taylor DJ, Tarnow-mordi W. Oracle Collaborative Group. Broad spectrum antibiotics for preterm prelabour rupture of fetal membranes- the oracle I randomized trial. Lancet 2001; 357: 979-88.
-
Gopalani S, krohn M, Meyn L, Hitti J, crombleholme WR. Contemporary management of preterm premature rupture of membranes: determinants of latency and neonatal outcome. Am J perinatol 2004; 21:183-90.
-
Yoon BH, Kim Ya, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature Rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J obstet Gynecol 1999; 181:784-788.
-
Lajos GJ, Passini Jr R, Nomura ML, Amaral E, Pereira BG, Milanez H, et al. Cervical bacterial colonization in women with preterm labor or premature rupture of membranes. Rev Bras Ginecol Obstet 2008; 30:393-399.
-
Cunningham FG, Leveno KJ , Bloom SL , Hauth JC, Gilstrapl, Wenstrom KD. Williams obstetrics . 22th ed. New york . MC Graw lioll; 2005; 232-247.
-
Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Degree of oligohydramnios and pregnancy outcome in patients with PROM. Obstet Gynecol 1985; 66:162-167.
-
Park JS, Yoon BH, Romero R, Moon JB, Oh SY, Kim JC, et al. The relationship between oligohydramnios and the onset of preterm labor in preterm premature rupture of membranes. AM J obstet Gynecol 2001; 184:459-462.
-
Mercer BM, Rabello YA, Thurnau GR, Miodovnik M, Goldenberg RL, Das AF, et al. the NICHD-MFMU antibiotic treatment of preterm PROM study: Impact of initial amniotic fluid volume on pregnancy outcome. Am J Obstet Gynecol 2006; 194:438-445.
-
Osmanagaoglu MA, Unal S, Bozkaya H. Chorioamnionitis risk and neonatal outcome in preterm premature rupture of membranes. Arch Gynecol Obstet 2005; 271:33-39.
-
Feinstein SJ, Vintzileos AM, lodeiro JG, Campbell WA, Weinbaum PJ, Nochimson DJ. Amniocentesis with premature rupture of membranes. Obstet Gynecol 1986; 68:147-152.
-
Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martins neonatal perinatal medicine. 2006; 1097-1101, 791-800.
-
Behrman, Kliegman, Jenson. Nelson text book of pediatrics. 2004; 573-586, 638.
-
Piazze J, Anceschi MM, Cerekja A, Brunelli R, Meloni P, Marzano s, et al. Validity of amniotic Fluid index in preterm rupture of membranes. J Perinat Med 2007; 35:394-398.
-
Borna S, Borna H, Khazardoost S, Hantoushzadeh S. Perinatal outcome in preterm premature rupture of membranes with Amniotic fluid index <5 (AFI<5). BMC Pregnancy Childbirth 2004; 4:15.
-
Moberg IJ, Garete TJ, Freeman RK. Fetal heart rate patterns of Fetal distress inpatient with prom. Obstet Gynecol 1984; 64:60-64.
-
Maymon E, Chaim W, Sheiner E, Mazor M. A review of randomized clinical trials of antibiotic therapy in preterm premature rupture of the membranes. Arch Gynecol Obstet 1988; 261:173-181.
-
Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003; 101:178-193.
-
Vermillion ST, Kooba AM, Soper DE. Amniotic Fluid index value after preterm PROM and subsequent perinatal infection. AM J Obstet Gynecol 2000; 183:271-276.
-
Sims EJ, Vermillion ST, Soper DE. Preterm premature rupture of the membranes is associated with a reduction in neonatal respiratory distress syndrome. Am J Obstet Gynecol 2002; 187:268-272.
-
Gonik B, Bottoms SF, Cotton DB. Amniotic fluid volume as a risk factor in preterm premature rupture of the membranes. Obstet Gynecol 1985; 65:456-459.