Study of Omentin1 and Other Adipokines and Hormones in PCOS Patients
Atheer Mahde, Mahmud Shaker, Zohair Al-Mashhadani
Mahde A, et al. OMJ. 24, 108-118 (2009); doi:10.5001/omj.2009.25
ABSTRACT
Objectives: Polycystic ovary syndrome (PCOS) is associated with insulin resistance and obesity. Recent studies have shown that plasma omentin-1 levels decrease with obesity. Currently, no data exists on the relative correlation between omentin-1 with other adipokines or the expression and regulation of omentin-1 in the serum of women with PCOS. The objective of this study is to evaluate the role of omentin-1 levels or omentin-1 /adipokines ratio in the serum of women with PCOS compared with matched control subjects.
Methods: The study involved 60 patients with PCOS and 30 women without PCOS who were used as controls. To examine the relationship between fasting serum omentin-1 and serum interleukin-6 (IL-6), interleukin-8 (IL-8), resistin, ghrelin, leptin RBP-4 and tumor necrosis factor-a (TNF-a) levels in infertile PCOS and non-PCOS subjects. Also, insulin and other hormones were measured in both groups. All these factors were measured by enzyme-linked immunosorbent assays.
Results: From the total of 60 cases, there was a significant increase (p<0.001) in PCOS patients when compared to the control group in fasting serum, serum interleukin-6 (IL-6), interleukin-8 (IL-8), resistin, leptin RBP-4, tumor necrosis factor-a (TNF-a) levels and insulin. A significant decrease in omentin-1 and ghrelin (p<0.001) was observed. The results also showed that 93.33% and 98.30% in PCOS patients had abnormal omentin1: Insulin ratio and omentin1: Resistin ratio respectively according to the cut off values ≤27.42 and ≤31.35. Moreover, 81.67 % of PCOS patients had abnormal omentin1:IL-6 ratio according to the cut of value (≤66.09).
Conclusion: This is the first time the role of plasma omentin1 has been investigated with respect to its implication in PCOS. Usually, LH/FSH ratio and FAI (ratio of total testosterone to SHBG) are the important factors used for the diagnosis of PCOS in all previous literature, but none of them referred to the parameters discussed in this report. Also, the percentage of sensitivity and the difference between range of these parameters in PCOS patients and the controls may give a different perspective in trying to understand the etiology of PCOS. Therefore, these parameters may be used for future diagnosis of PCOS. This study also suggested that omentin/resistin ratio may play a crucial paracrine or endocrine role in modulating insulin sensitivity.
From the Department of Acceptable Analysis, Health and Medical Technical College, Baghdad, Iraq.
Received: 07 Jan 2009
Accepted: 27 Feb 2009
Address correspondence and reprint request to: Dr Atheer Mehdi, Department of Acceptable Analysis, Health and Medical Technical College, Baghdad, Iraq.
E-mail: atheerawod@yahoo.com
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrine metabolic disease that occurs in 5-10% of women of reproductive age.1-3 PCOS is an inherited disease that affects women of childbearing age.4
The metabolic syndrome is associated with excessive accumulation of central body fat. As well as its role in energy storage, adipose tissue produces several hormones and cytokines termed adipokines that have widespread effects on carbohydrate and lipid metabolism. They appear to play an important role in the pathogenesis of insulin resistance (IR), diabetes, and atherosclerosis.5 Adipose tissue is now hypothesized to be the largest endocrine organ in the body, secreting a large number of biologically important substances termed adipokines. The best characterized adipokines are adiponectin, leptin, TNF-a, and IL-6.6 Other identified adipokines include Plasminogen activator inhibitor-1 (PAI-1), resistin, visfatin and glucocorticoids.6
These adipokines are postulated as a potential link between abdominal obesity and the vasculature, and have been shown to mediate IR.7 Numerous studies have also found associations between IR and increased TNF-a, IL-6, macrophages and monocyte chemoattractant protein-1 (MCP-1), in addition to PAI-1 , adipsin, and decreased adiponectin.8
Omentin1 (also named Omentin, Intelectin-1, Endothelial Lectin HL-1 and Intestinal Lactoferrin Receptor) has been identified as a major visceral (omental) fat secretory adipokine.9The mature omentin is a secretory glycoprotein consisting of 295 amino acids and N-linked oligosaccharides, and its basic structural unit is a 120-kDa homotrimer in which 40-kDa polypeptides are bridged by disulfide bonds. It is secreted by omental adipose tissue, much less in the intestines, lung and heart and highly abundant in human plasma.9
Omentin-1 is a new type of Ca2+-dependent lectin with affinity for galacto furanosyl residues (constituents of pathogens and dominant inmunogens).10 It was suggested, therefore, that a biological function of omentin/intelectin was the specific recognition of pathogens and bacterial components, an important role in the innate immune response to parasitic infections.11 Moreover, several studies have shown that omentin gene expression is altered by inflammatory states and obesity.12
Kuperman et al (2005) found increased gene expression of omentin in airway epithelial cells of patients with asthma.13 Intriguingly, differential expression of omentin mRNA occurs in omental adipose tissue of patients with Crohn’s disease, suggesting that omentin could be a new candidate factor potentially involved in chronic inflammatory diseases in humans.10
A homolog of omentin has been identified that it shares 83% amino acid identity with omentin/intelectin and it may be referred to as omentin-2. 14 The two omentin genes, omentin-1 and omentin-2, are localized adjacent to each other in the 1q22-q23 chromosomal region, which has been previously linked to Type II Diabetes Mellitus in several populations. 15 ,16-19
In vitro studies have shown that omentin increases insulin signal transduction by activating the protein kinase Akt/protein kinase B and enhancing insulin-stimulated glucose transport in isolated human adipocytes.9
Lean subjects had significantly higher plasma omentin levels than obese and overweight subjects. In addition, higher plasma omentin levels were detected in women compared with men.20
Omentin-1 is decreased in patients with PCOS, a disease associated with IR and obesity.21 Glucose and insulin negatively regulate Omentin1 levels ex vivo and in vivo.21 Therefore, omentin levels may be predictive of the metabolic consequences or co-morbidities associated with obesity.
METHODS
Subjects
Samples
After fasting overnight, the venous blood samples were aspirated at 08:00-10:00 am during the 3rd-6th day of the menstrual cycle (early follicular phase) for patients with normal cycles. For patients with ovulation or oligomenorrhea, blood samples were collected regardless of the duration of the cycle. The samples were transferred into clean, plain tubes and centrifuged within 30 minutes of collection. Then the serum from all blood samples were separated; sugar and lipid profile levels were measured using enzyme colorimetric methods and the rest were stored at 18°C until the time of assay.
Patients
60 infertile Iraqi women aged 18-37 years (M±SD: 26.45±4.65) were enrolled for the study. Their medical histories were noted, body weight and height were measured, and body mass index (BMI) was calculated [mean BMI (34.01±3.54 Kg/m2)]. PCOS patients had already been diagnosed and the diagnosis was confirmed according to the European society of human reproduction and embryology and American society for reproductive medicine criteria; PCOS is diagnosed if there are any two of the following:
• Presence of polycystic ovary on ultrasound examination.
• Clinical or biochemical hyperandrogenemia.
• Menstrual dysfunction with an ovulation.
Healthy Control Group
For comparison, thirty apparently healthy fertile women with regular cycles who matched for age, weight, and BMI [n=30; age=28.87± 3.27 (years); BMI=29.56 ± 2.12 (kg/m2); mean ± SD] were also included as control subjects.
Protocol
Clinical and anthropometrical variables, including clinical blood pressure, BMI, (calculated as kg/m2) and waist-to-hip ratio (WHR) were determined in all the subjects. Omentin-1, IL6, IL-18, resistin, TNF-a, ghrelin, RBP-4 leptin, insulin, LH, FSH, total testosterone, free testosterone and sex hormone-binding globulin (SHBG) were determined by ELISA methods. Serum fasting glucose level was measured by enzymatic method supplied by Giesse Diagnostics.
The homeostasis model assessment (HOMA) was used to calculate an index of insulin resistance for each patient, and beta-cell function was estimated from fasting insulin and glucose levels by the homeostasis model assessment (HOMA-b). Using the fasting glucose (mmol/L) and insulin (µIU/ ml), the index for insulin resistance; HOMA IR, was defined as (insulin ×glucose)/22.5 and HOMA b-cell function × 20 × insulin/(glucose-3.5).22.
Statistics
All statistical analysis in the study were performed using SPSS version 15.0 for Windows (Statistical Package for Social Science, Inc., Chicago, IL, USA). Descriptive analysis was used to show the mean and standard deviation of the variables.
The significant difference between mean values was estimated by the Student t-test. The point of statistical significance was noted when probability was p<0.05, and no statistical significance was noted when p>0.05. Correlation analysis was used to test the linear relationship between parameters. ANOVA test was used to show the differences between variables of differentiated groups.
RESULT
Compared with normal controls, the results showed that women with PCOS had increased (p<0.001) levels of IL-6, IL-18, TNF-a, RBP-4, Resistin, leptin, insulin, LH, testosterone, free testosterone, HOMA, and HOMA ß-cell% (table 1). Furthermore, patients with PCOS also had lower omentin-1 (p<0.001), ghrelin(p<0.05) and QUICKI (p<0.001) values than controls (table 1), while FSH and FBG levels were not significantly different (p>0.05) between the two groups. The PCOS patients were divided into 2 groups. Group 1; thirty one PCOS patients with irregular menstrual cycles (oligomenorrhea, amenorrhea) and group 2; twenty nine PCOS patients with regular menstrual cycles (table 2).
Table 1: Clinical and baseline Adipokines, hormone concentrations in PCOS and control groups.
Characteristic |
PCOS mean ± SD |
Control mean ± SD |
p (t-test) |
BMI Kg/m2 |
33.14±1.88 |
29.56±1.01 |
<0.001 |
WHR |
0.86±0.02 |
0.80±0.02 |
<0.001 |
TNF-a pg/mlPg / |
5.56 ±1.53 |
2.98±0.51 |
<0.001 |
IL-6 pg/ml |
5.82±1.66 |
2.75±0.66 |
<0.001 |
IL-18 pg/ml |
298.98±107.34 |
229.60±55.34 |
<0.001 |
RBP-4 µg/ml |
35.11±6.51 |
27.33±3.49 |
<0.001 |
Resistin ng/ml |
13.78±2.26 |
7.66±0.66 |
<0.001 |
Leptin ng/ml |
31.92 ± 2.39 |
28.08 ± 1.63 |
<0.001 |
Ghrelin pg/ml |
138.22±58.40 |
169.67±25.51 |
<0.05 |
Omentin-1 ng/ml |
252.45±33.39 |
327.50±40.34 |
<0.001 |
Insulin µU/ml |
20.93 ±8.38 |
8.05±0.64 |
<0.001 |
FBG mg/dl |
86.0±7.00 |
85.57±6.39 |
>0.05 |
HOMA |
4.61 ±2.06 |
1.58±0.16 |
<0.001 |
HOMA ß-cell% |
375.90 ±194.70 |
132.86±33.48 |
<0.001 |
QUICKI |
0.31 ±0.02 |
0.35±0.01 |
<0.001 |
LH IU/L |
14.91 ±2.68 |
9.53±1.77 |
<0.001 |
FSH IU/L |
5.20 ±0.71 |
5.54±0.85 |
>0.05 |
Testosterone nmole/L |
2.66 ±0.40 |
2.18±0.27 |
<0.001 |
Free testosterone pg/ml |
3.87 ±1.24 |
2.12±0.49 |
<0.001 |
SHBG nmole/L |
5.25 ±3.72 3 |
53.06±4.95 |
<0.001 |
FAI % |
7.98 ±0.85 |
5.19±1.33 |
<0.001 |
PCOS: Polycystic ovary syndrome; BMI: Body mass index; WHR: Waist-to-hip ratio; TNF: Tumor necrosis factor; IL: Interleukin; RBP: Retinol binding protien; FBG: Fasting blood suhar; HOMA: Homeostasis model assessment; LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; SHBG: Sex hormone-binding globulin; FAI: Free androgen index; QUICKI: Quantitative insulin-sensitivy check index |
|||
Table 2: Clinical and baseline Adipokines, hormone concentrations in PCOS with irregular and regular menstrual cycle
Characteristic |
PCOS (Irregular menstrual cycle) mean ± SD |
PCOS (Regular menstrual cycle) mean ± SD |
p (t-test) |
|
BMI Kg/m2 |
33.87±1.66 |
32.36±1.81 |
<0.001 |
|
WHR |
0.87±0.02 |
0.86±0.02 |
>0.05 |
|
TNF-a pg/mlPg / |
6.47 ±1.21 |
4.58±1.211 |
<0.001 |
|
IL-6 pg/ml |
6.37±1.68 |
5.23±1.54 |
<0.001 |
|
IL-18 pg/ml |
361.42±79.99 |
232.24±92.15 |
<0.001 |
|
|
|
|
|
|
RBP-4 µg/ml |
36.15±6.14 |
33.86±5.26 |
>0.05 |
|
Resistin ng/ml |
14.84±2.22 |
12.66±1.70 |
<0.001 |
|
Leptin ng/ml |
32.83 ± 1.94 |
30.95 ± 2.47 |
<0.001 |
|
Ghrelin pg/ml |
108.39±49.96 |
160.10±49.72 |
<0.05 |
|
Omentin-1 ng/ml |
237.32±23.42 |
260.24±57.00 |
<0.001 |
|
Insulin µU/ml |
25.38 ±7.88 |
16.17±6.00 |
<0.001 |
|
F.BG mg/dl |
87.68±8.11 |
85.44±5.50 |
>0.05 |
|
HOMA |
5.69 ±1.96 |
3.46±1.47 |
<0.001 |
|
HOMA ß-cell% |
455.00 ±207.00 |
291.34±140.13 |
<0.001 |
|
QUICKI |
0.30 ±0.01 |
0.32±0.01 |
<0.001 |
|
LH IU/L |
14.68 ±2.34 |
15.16±3.03 |
>0.05 |
|
FSH IU/L |
5.17 ±0.77 |
5.23±0.66 |
>0.05 |
|
Testosterone nmole/L |
2.65 ±0.35 |
2.64±0.37 |
>0.05 |
|
Free testosterone pg/ml |
3.81 ±1.37 |
3.94±1.10 |
>0.05 |
|
SHBG nmole/L |
5.57 ±3.79 3 |
34.91±3.68 |
>0.05 |
|
FAI % |
7.93 ±0.94 |
8.02±0.76 |
>0.05 |
|
|
|
|
|
|
|
||||
There was a negative correlation in Omentin-1[ng/ml] with BMI (r=-0.435, p<0.001), insulin (r=-0.424, p<0.001), HOMA (r=-0.415, p<0.001), HOMA ß-cell% (r=-0.358, p<0.001), LDL-cholesterol (r=-0.328, p<0.05), IL18 (r=-0.411, p<0.001), IL6 (r=-0.458, p<0.001), TNF-a (r=-0.431, p<0.001), RBP4 (r=-0.378, p<0.05), resistin (r=-0.428, p<0.001), and leptin (r=-0.370, p<0.01) between the PCOS group and the control group. A significant positive correlation was found between omentin1 (ng/ml), and QUICKI ([r=0.497, and p<0.001), ghrelin (r= 0.511, p<0.001) as shown in table 3.
Table 4 shows a number of parameters which depend on cut-off values (values above or below the mean ± 2SD) and according to them, sensitivity could be calculated as shown in table 5 and figure 1, 2 respectively. Table 5 figure 1 and 2 show the cut-off values (values ± 2 SD of the mean) from which sensitivity was calculated.
In this study, a significantly negative association between omentin-1/resistin and insulin ratio was found in PCOS patients while there was no significant correlation between insulin and ominten in the control group (figure 3).
Table 3: Baseline Pearson correlations coefficients of omentin1 levels with various metabolic and hormonal parameters in patients with PCOS.
Variable |
Omentin1 |
|
Correlation |
p value |
|
BMI (Kg/m2) |
-0.435 |
0.001> |
Fasting insulin (μU/ml) |
-0.424 |
0.001> |
HOMA |
-0.415 |
0.001> |
HOMA ß-cell% |
-0.358 |
0.05> |
QUICKI |
-0.497 |
0.001> |
Ghrelin (pg/ml) |
-0.511 |
0.001> |
IL18 (pg/ml) |
-0.411 |
0.001> |
IL6 (pg/ml) |
-0.458 |
0.001> |
TNF-a (pg/ml) |
-0.431 |
0.001> |
RBP4 (µg/ml) |
-0.378 |
0.05> |
Resistin (ng/ml) |
-0.428 |
0.001> |
Leptin (ng/ml) |
-0.370 |
0.01> |
BMI: Body mass index; HOMA: Homeostasis model assessment; IL: Interleukin; TNF: Tumor necrosis factor; QUICKI: Quantitative insulin-sensitivy check index; RBP: Retinol binding protien |
||
Table 4: Omentin1 ratios in PCOS patients compared to control.
p |
Control [n=30] Mean± SD |
PCOS [n=60 Mean± SD] |
Characteristic |
<0.0001 |
40.98±6.78 |
14.57±7.44 |
omentin1 : Insulin ratio |
<0.0001 |
1.52±0.30 |
1.02±0.54 |
omentin1 : IL18 ratio |
<0.001 |
126.99±30.4 |
48.11±18.33 |
omentin1: IL6 ratio |
<0.0001 |
112.41±22.32 |
50.78±22.59 |
omentin1 : TNF-a ratio |
<0.0001 |
13.17±2.25 |
7.53±2.08 |
omentin1 :RBP4 ratio |
<0.0001 |
42.89±5.77 |
19.05±5.26 |
omentin1 :Resistin ratio |
<0.0001 |
11.26±1.18 |
7.99±1.55 |
omentin1 :Leptin ratio |
<0.0001 |
209.25±38.11 |
68.91±38.01 |
omentin1 :HOMA ratio |
<0.0001 |
2.61±0.70 |
0.87±0.50 |
omentin1 : HOMA ß-cell% ratio |
Table 5: The sensitivity of omentin1 ratios in PCOS.
Sensitivity |
Test |
93.33% |
omentin1 : Insulin ratio ≤27.42 |
55.00 % |
omentin1 : IL18 ratio ≤0.92 |
81.67 % |
omentin1 : IL6 ratio ≤ 66.09 |
81.67% |
omentin1 : TNF-a ratio ≤67.77 |
71.67 % |
omentin1 :RBP4 ratio ≤8.67 |
98.30 % |
omentin1 :Resistin ratio ≤31.35 |
53.00 % |
omentin1 :Leptin ratio ≤8.90 |
95.00 % |
omentin1 :HOMA ratio ≤133.03 |
86.67 % |
omentin1: HOMA ß-cell% ratio ≤1.21 |
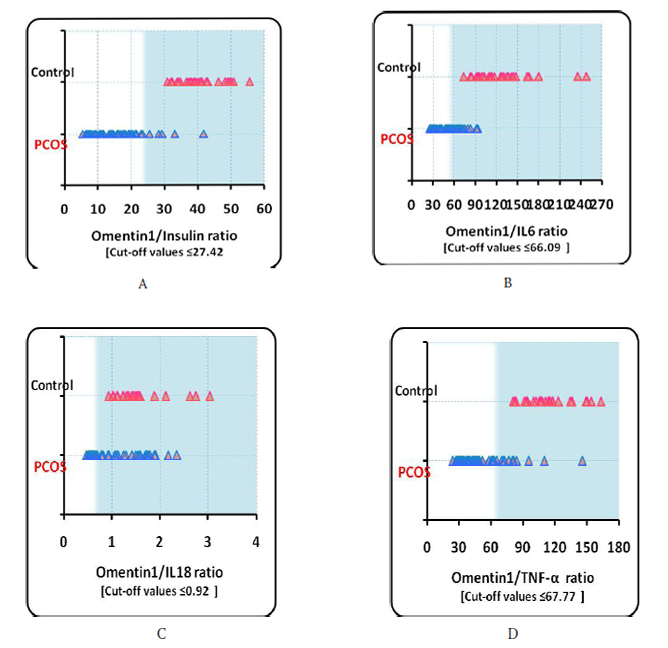
Figure 1: Values for Omentin1/Insulin ratio, Omentin1/IL6 ratio, Omentin1/IL18 ratio and Omentin1/TNF-a ratio (from A to D)
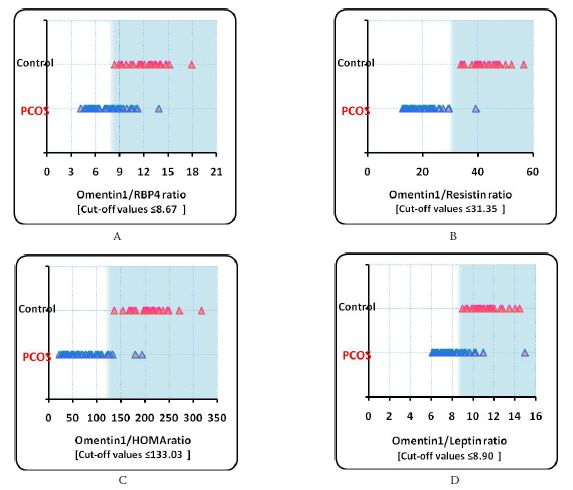
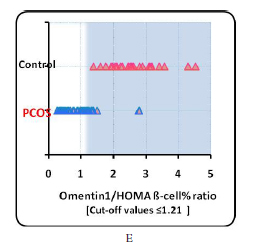
Figure 2: Values for Omentin1/RBP4 ratio, Omentin1/Resistin ratio, Omentin1/Leptin ratio, Omentin1/HOMA ratio and Omentin1/HOMA ß-cell (from A-E)
In this study, a significantly negative association was observed between omentin-1/resistin ratio and insulin in the PCOS patients while there was no significant correlation was observe in the control group.
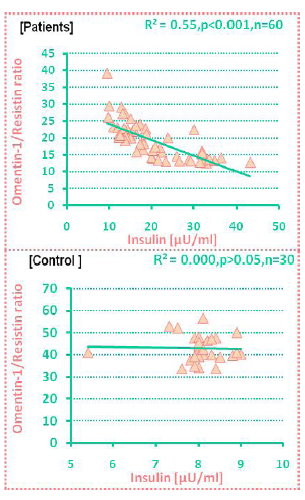
Figure 3. Correlation between omentin/resistin ratio and insulin in PCOS patients and in control.
In this study, a significantly inverse association between omentin-1 /IL-6 ratio and insulin was found in PCOS patients while there was no significant correlation between them in control as shown figure 4.
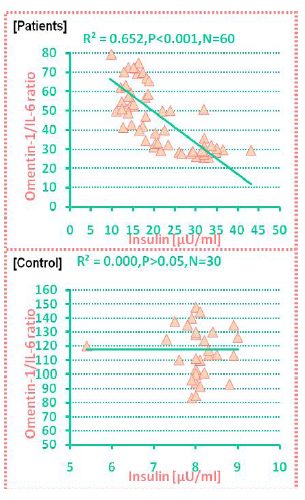
Figure 4. Correlation between omentin-1/IL-6 and insulin in PCOS patients and in control.
DISCUSSION
Obesity is one of the clinical characteristics of the PCOS along with oligomenorrhea, hirsutism, and infertility. However, not all obese females have PCOS and not all PCOS patients are obese. This study also showed a significant increase (p<0.001) in BMI and WHR in PCOS patients (table 1). The cause of obesity in the PCOS patients remains unknown, however, it is thought that obesity may play a pathogenetic role in the development of the syndrome in susceptible individuals.23,24 The study showed that women with PCOS in Iraq have a higher body weight than their counterparts.25-27 This maybe as a result of different nutritional habits in Iraq than other countries.
26.67% of PCOS patients had normal TNF-a values (≥4.00 Pg/ml). Serum levels of TNF-a had been found to be elevated in patients with PCOS compared with age- and/or BMI in the controlled group, these findings are also consistent with several other studies.28-31
There was a significant difference (p<0.001) in IL-6 between regular menstrual cycle and irregular menstrual cycle in PCOS patients (table 2). This finding is suggestive of a physiologic modulation of cytokine secretion by the pituitary hormones, although in this study protocol, the gonadotropin stimulation was not observe at any pharmacological dosage. Indeed, previous studies have demonstrated that FSH was able to induce IL-6 expression from granulosa cells, and a possible regulation of IL-6 expression by LH has also been suggested.32,33 The increase levels of IL-6 could be in relation to the angiogenesis occurring during the ovarian follicular development.34 In PCOS patients, the intra follicular IL-6 and TNF-a concentrations were higher than those found in the serum, suggesting a local production of these cytokines. Indeed, even if both cytokines are mainly produced by white blood cells, they can also be produced by granulosa cells.35 The mean level of IL-18 was significantly elevated in the PCOS patients compared to the control group [p>0.001] as shown in table 1, a result which is compatible with other studies, while another study did not find any statistical difference between PCOS and the control group.36-38 The increase in IL-18 levels found in the PCOS patients was probably related to the visceral adiposity and insulin resistance, frequently found in PCOS patients.39
Serum RBP-4 was found to be significantly elevated (p<0.0001) in PCOS patients with a mean of 35.11±6.51 μg/ml compared to the control group with a mean of 27.33±3.49 μg/ml as shown in table 1. Elevated serum levels of RBP-4 in PCOS were demonstrated in several studies, but another reported that mean basal levels were decreased in PCOS and in the control group.40-43 There was no significant difference (p≥0.05) between the two subgroups of PCOS women according to their menstrual cycle, (table 2), this may be due to the fact that RBP-4 concentrations were not correlated with biochemical or clinical endocrine parameters.44
The altered levels of resistin may be the consequence of altered adipose tissue function but also as a result of a difference in fat distribution in women with PCOS, because they tend to have proportionally more visceral adipose tissue.45 In fact, it has been suggested that differences in adipose tissue distribution may influence the secretion of the different adipocytokines.46
Some studies have found that there was no difference in leptin levels between PCOS and control women.47,48 Other studies however, found leptin levels in women with PCOS to be higher than expected for their BMI, suggesting abnormalities in leptin signaling to the reproductive system in PCOS.49-52 A study by Basma suggested that leptin was lower in PCOS patients.53 The differences in the results may be due to different patient number and BMIs. There was a significant difference (p<0.001) in leptin between regular menstrual cycle and irregular menstrual cycle in PCOS patients, (table 2), This finding may be due to the fact that Leptin, a nutrition-and adiposity-dependent protein with a potential to modulate the gonadotropin axis in several mammals, has been proposed to be a possible link between energy metabolism and ovarian function.54 Some studies in the literature have suggested that leptin affects fertility through its central and peripheral effects.55 Leptin levels are not constant and may vary with follicular growth and maturation and thus fluctuate throughout the menstrual cycle.56 Additionally, nocturnal changes in luteinizing hormone pulse parameters are associated temporarily with the rise of plasma concentrations of leptin at night, and the fluctuations of plasma leptin concentrations are synchronous with those of LH and estradiol.57
Data on ghrelin levels in women with PCOS are rather conflicting: both decreased, and elevated concentrations have been reported, while others have found no significant differences between women with the syndrome and normal women. 58- 62 There was a significant difference (p<0.001) in gherlin between regular menstrual cycle and irregular menstrual cycle in PCOS patients, (table 2), this may be due to the fact that ghrelin is a hormone that can cause reduced secretion of hormones which regulate ovarian and menstrual functions.63
Visceral obesity is highly associated with risks of type II diabetes, cardiovascular disease, hypertension, and hyperlipidemia.5,64,65 Expression of adipose-derived factors such as leptin, TNF-a, RBP4, and resistin are modulated by obesity.66-69 Therefore, this study of circulating omentin levels was undertaken to better understand the regulation and role of omentin1, a new visceral fat depot-specific adipokine, in obesity and insulin resistance in PCOS patients. In Iraq, no previous study has clarified the role of omentin1 in PCOS patients or investigated the relationship between omentin and other parameters.
Serum omentin-1 was found to be significantly decreased (p<0.0001) in PCOS patients with a mean of 252.45±33.39 ng/ml compared to controls with a mean of 327.50±40.04 ng/ml (table 1). Decreased serum levels of omentin-1 in PCOS were demonstrated in one study only. 53.33% of patients with PCOS had abnormal omentin-1 values according to the cut-off value ≤ 247.42 ng/ml.21 There was a significant difference (p<0.001) in omentin-1 between regular menstrual cycle and irregular menstrual cycle in PCOS patients (table 2), this may be due to the fact that omentin1 can affect the secretion of hormones that regulate ovarian and menstrual function or affect receptors for these hormone. This study suggests that more detailed research should be conducted to evaluate the role of novel peptides in women with PCOS.
There is evidence to suggest that omentin-1 may be involved in some aspect of insulin sensitivity, and there was a negative correlation between omentin1 plasma levels and measurements of insulin resistance (HOMA), fasting insulin, and HOMA ß-cell%, but there was a positive correlation with QUICKI and ghrelin. Moreover, negative correlations between plasma omentin-1 levels and BMI, resistin, IL6, IL18, TNF-a and leptin values were observed. There was a negative association between plasma omentin-1 with these metabolic indices suggesting that higher omentin-1 levels may be seen as a marker for leanness or as a positive factor that opposes the obese state and its pathophysiological consequences. Also the data suggests that some aspects of obesity negatively regulates omentin1 expression and release it into the circulation. Although the data clearly supports the regulation of omentin by obesity, omentin-1 may also be regulated by inflammation. Other studies have shown that omentin-1 expression is altered in inflammatory states.10,13 Obesity itself is associated with low levels of chronic inflammation, which may contribute to the regulation in the role of omentin in human physiology.70,71 Consequently, weight loss and different inflammatory states could be modulators of omentin-1 expression and function. In summary, there is evidence to suggest that plasma omentin-1 is inversely related to obesity.
In Iraq, there was no study which showed these correlations and this study suggests that omintin1 is a good index to confirm IR.
The physiological role of omentin-1 in glucose metabolism, omentin’s target tissues, a receptor, or relevant signal transduction pathways are still to be determined.
In this study, several parameters that depend on the omentin1 ratios, (table 4) were observed. Cut-off values determined the sensitivity. These findings are detected for the first time, while LH/FSH ratio and FAI (ratio of total testosterone to SHBG) are the usually important factors used for diagnosis of PCOS in all literature review but none of them referred to present parameters, the good percentage of sensitivity and also the difference between the range of parameters in PCOS and controls may give a different perspective in order to understand the etiology of PCOS. Therefore, the parameters may be useful in determining the diagnosis of PCOS.
In this study, a significantly negative association between omentin-1 / resistin ratio and insulin was found in PCOS patients while there was no significant correlation between them in the control group (figure 3). In vitro studies have shown that omentin increases insulin signal transduction by activating the protein kinase Akt/protein kinase B and enhancing insulin-stimulated glucose transport in isolated human adipocytes.10 This study suggested that omentin-1/resistin ratio may play a paracrine or endocrine role in modulating insulin sensitivity.
In this study, a significantly inverse association between omentin-1/IL6 ratio and insulin was found in PCOS patients while there was no significant correlation between them in the control group (figure 4).
Serum IL-6 concentration was positively correlated with obesity and insulin resistance and IL6 expression in adipose tissue with obesity.70,71 This study tentatively hypothesized that the decreased omentin-1/IL-6 ratio levels in the serum of women with PCOS as demonstrated in this study may partly explain the corresponding increased levels of insulin observed in the serum of women with PCOS, possibly as a consequence of the hyperinsulinemic state seen in these women. However, these are hypotheses which need to be further investigated. It is important to bear in mind that the regulation of omentin-1 in adipose tissue is probably multifactorial. Moreover, it would be of interest to know whether or not the effects of insulin on omentin-1 production are also applicable to other tissues given the in vivo results observed in this study. Hence, further studies are needed to elucidate the role of other factors that regulate omentin-1 production.
CONCLUSION
The findings detected in this study have been recorded for the first time, while LH/FSH ratio and FAI (ratio of total testosterone to SHBG) are usually the important factors used for the diagnosis of PCOS in all literature review but none of them referred to present parameters, the % of sensitivity and also the difference between the range of parameters in PCOS patients and control group may give a different perspective in understanding the etiology of PCOS. Thus these parameters can be used for the diagnosis of PCOS. The study also showed that omentin/resistin ratio may play a paracrine or endocrine role in modulating insulin sensitivity.
ACKNOWLEDGMENTS
The authors reported no conflict of interest and no funding has been received on this work.
1.Azziz R. Polycystic ovary syndrome is a family affair. J Clin Endocrinol Metab 008; 93:1579-1581.
2.Mueller A, Gooren LJ, Naton-Schotz, Cupisti S, Bechman MW, Dittrich R. Prevalence of polycystic ovary syndrome and hyperandrogenemia in female-to-male transsexuals. J Clin Endocrinol Metab 2008; 93:1408-1411.
3.Codner E, Escobar-Morreale HF. Clinical review: Hyperandrogenism and polycystic ovary syndrome in women with type 1 diabetes mellitus. J Clin Endocrinol Metab 2007; 92:1209-1216.
4. Martha J, Frances J, Patrick M, Judith M, Clay R, Jouko I. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome. Annals of Neurology 2008; 64:200-211.
5.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89:2548-2556.
6.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern 2004; 164:1066-1076.
7.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab 2004; 89:447-452.
8.Sampson M, Kong C, Patel A, Unwin R, Jacobs HS. Ambulatory blood pressure profiles and plasminogen activator inhibitor (PAI-1) activity in lean women with and without the polycystic ovary syndrome. Clinical Endocrinology 1996; 45:623-629.
9.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am J Phys Endocrinol Metab 2006; 290: 1253-1261.
10.Schaffler A. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta 2005; 1732:96–102.
11.Gerwick L. Gene transcript changes in individual rainbow trout livers following an inflammatory stimulus. Fish Shellfish Immunol 2007; 22:157-171.
12.de Souza Batista CM. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007; 56:1655-1661.
13.Kuperman DA. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 2005; 116:305-311.
14.Lee JK, Schnee J, Pang M, Wolfert M, Baum LG, Moremen KW, et al. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology 2001; 11:65-73.
15.Fu M, Gong DW, Damcott C, Sabra M, Yang RZ, Pollin TI, et al. Systematic analysis of omentin 1 and omentin 2 on 1q23 as candidate genes for type 2 diabetes in the Old Order Amish. Diabetes 2004; 53:59.
16.Elbein SC, Hoffman MD, Teng K, Leppert MF, Hasstedt SJ. A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes 1999; 48:1175-1182.
17.St Jean P, Husueh WC, Mitchell B, Ehm M, Wanger M, Burns D, et al. Association between diabetes, obesity, glucose and insulin levels in the Old Older Amish and SNPs on 1q21-23. Am J Hum Genet 2000; 67:332.
18.Vionnet N, Hani El H, Dupont S, Gallina S, Francke S, Dotte S, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: Evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet 2000; 67:1470-1480.
19.Wiltshire S, Hattersley AT, Hitman GA, Walker M, Levy JC, Sampson M, et al. A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): Analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q; Am J Hum Genet 2001; 69:553-569.
20.Celia M, Rong-Ze Y, Mi-Jeong L, Nicole M, Dao-Zhan Y, Jessica P, et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007; 56:1655-1661.
21.Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, et al. Omentin-1, a novel adipokine, is decreased in Overweight insulin resistant women with the polycystic ovary syndrome: Ex vivo and in vivo Regulation of Omentin-1 by Insulin and Glucose; Diabetes 2008; 57:801-808.
22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412-419.
23.Escobar H, Morreale F. The Polycystic ovary syndrome associated with obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2005; 27:12-15.
24. Ronald S, Beth Y, Arthur F. In “Danforths obstetrics and gynecology; 9th edition. Lippincott Williams & Wilikins. Philadelphia. 2003 p. 663-683.
25.Azziz R, Ehrmann D, Legro R. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: A multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2001; 86:1626-1632.
26.Conway G, Honour J, Jacobs H. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol (Oxf) 1998; 30:459-470.
27.Carmina E, Legro R, Stamets K, Lowell J, Lobo R. Difference in body weight between American and Italian women with polycystic ovary syndrome: Influence of the diet. Hum Reprod 2003; 18:2289-2293.
28.Gonzalez F, Thusu K, Abdel−Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor−a in normal−weight women with polycystic ovary syndrome. Metabolism 1999; 48:437-441.
29.Sayin NC, Gucer F, Balkanli−Kaplan P, Yuce MA, Ciftci S, Kucuk M, et al. Elevated serum TNF−alpha in normal−weight women with polycystic ovaries or the polycystic ovary syndrome. Reprod Med 2003; 48:165-170.
30.Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol 2003; 101:1177-1182.
31. Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003; 80:123-127.
32.Zolti M, Meirom R, Shemesh M, Wollach D, Mashiach S, Shore L, et al. Granulosa cells as source and target organfor tumor necrosis a. FEBS Lett 1990; 261:253-255.
33.Loret de Mola JR, Baumgardner GP, Goldfarb JM, Friedlander MA. Ovarian hyperstimulation syndrome: Preovulatory serum concentrations of interleukin-6, interleukin- 1 receptor antagonist and tumor necrosis factopr-alpha cannot predict its occurrence. Hum Reprod 1996; 11: 1377–1380.
34.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci 1990; 87:3092-3096.
35.Castilla JA, Sampaio A, Molina R, Samaniego F, Mozas J, Vergara F, et al. Mononuclear cell subpopulations in human follicular fluid from stimulated cycles. Am J Reprod Immunol 1990; 22:127-129.
36.Zhang Y, Yang Y, Hong J, Gu W, Shen C, Xu L, et al. Elevated serum levels of interleukin-18 are associated with insulin resistance in women with polycystic ovary syndrome. Endocrine 2006; 29: 3:419-423.
37.Mani R, Pulliangudi S, Selvi M, Raghunathan M. Cytokines and leptin correlation in patients with polycystic ovary syndrome: Biochemical evaluation in south Indian population. reproductive med biol 2005; 4:247-254.
38.Kowalska S, Nikolajuk A, Kozlowska A, Karczewska M, Adamska A, et al. Plasma interleukin 18 in patients with polycystic ovary syndrome: Relation to insulin resistance. Endocrine Abstracts 2006; 11:249.
39.he´ ctor f. escobar-morreale, jose´ i. botella-carretero, gemma villuendas, jose´ sancho, jose´ l. san milla´n. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: Relationship to insulin resistance and to obesity. J Clin Endocrinol Metab 2004; 89:806-811.
40.Hutchison SK, Harrison C, Stepto N, Meyer C, Teede HJ. Retinol-binding protein 4 and insulin resistance in polycystic ovary syndrome. Diabetes Care 2008; 31:1427-32.
41.Tan B, Jing C, Sheryl P, Richard KB, Randeva H. Raised serum, adipocyte and adipose tissue retinol binding protein 4 (RBP4) in women with polycystic ovary syndrome. Endocrine Abstracts 2007; 14:71.
42.Aigner E, Bachofner N, Klein K, De Geyter C, Hohla F, Patsch W, et al. Retinol-binding protein4 (RBP4) in polycystic ovary syndrome (PCOS): Association with steroid hormones and response to pioglitazone treatment. 2009; 21:23-26.
43.Diamanti-Kandarakis1 E,Livadas1 S, Kandarakis S, Papassotiriou I, Margeli A. Low free plasma levels of retinol-binding protein 4 in insulin-resistant subjects with polycystic ovary syndrome. J Endocrinol Invest 2008; 31:950-955.
44.Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, et al. Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. European Journal of Endocrinology 2007; 157:201-207.
45.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. Journal of Biological Chemistry 2002; 277:25863-25866.
46.Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, et al. Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice;Diabetes 2003; 52:2266-2273.
47.Caro JF. Leptin is normal in PCOS, an editorial about three “negative” papers. J Clin Endocrinol Metab 1997; 82:1685-1686.
48.Petermann T, PiwonkaV, Pe´rez F, MaliqueoM, Recabarren S, Wildt L. Are circulating leptin and luteinizing hormone synchronized in patients with polycystic ovary syndrome. Human Reproduction 1999; 14:1435-1439.
49. Vicennati V, Gambineri A, Calzoni F, Casimirri F, Macor C, Vettor R, et al. Serum leptin in obese women with polycyctic ovary syndrome is correlated with body weight and fat distribution but not with androgen and insulinlevels. Metabolism 1998; 47:988-992.
50.Rouru J, Anttila L, Koskinen P, Anttila L, Koskinen P, Tuula-Penttilä A, et al. Serum leptin concentrations in women with polycyctic ovary syndrome. J Clin Endocrinol Metab 1997; 82:1697-1700.
51.Nobumasa K, Kazumichi, Hideki M, Takashi M. Relationships between circulating leptin concentrations and other hormonal parameters in obese and non-obese women with polycystic ovary syndrome. Reprod Med Biol 2002; 1:49-54 .
52.Blagovest P, Mitko M. Serum leptin levels correlate with clinical and biochemical indices of insulin resistance in women with polycystic ovary syndrome. The European Journal of Contraception & Reproductive Health Care 2009; 14:153-159.
53.Mustafa B, Wafa R, Waleed R. Hormonal, biochemical, and ovarian response to aromatas inhibitors in clomiphene citrate resistant PCOS (comparative study): A thesis submitted to the department of the clinical laboratory analysis and the committee of graduate studies of college of pharmacy in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Pharmacy. 1997.
54.Cioffi J, Shafer A, Zupancic T, Smith-Gbur J, Mikhail A, Platika D. et al. Novel B 219/OB receptor isoforms: Possible role of leptin in hematopoiesis and reproduction. Nat Med 1996; 2:585-588.
55.Barash I, Cheung C, Weigle D, Ren H, Kabigting E, Kuijper J, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996; 137:3144-3147.
56.Gennarelli G, Holte J, Wide L, Berne C, Lithell H. Is there a role for leptin in the endocrine and metabolic aberrations of polycystic ovary syndrome? Hum Reprod 1998; 13:535-541.
57.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci 1998; 95:2541-2546.
58.Pagotto U, Gambineri A, Vicennati V, Heiman ML, Tschop M, Pasquali R. Plasma ghrelin, obesity, and the polycystic ovary syndrome, correlation with insulin resistance and androgen levels. J Clin Endocrinol Metab 2002; 87;5625-5629.
59.Schofl C, Horn R, Schill T, Schlosser HW, Muller MJ, Brabant G. Circulating ghrelin levels in patients with polycystic ovary syndrome;J Clin Endocrinol Metab 2002; 87:4607-4610.
60.Moran LJ, Noakes M, Clifton PM, Wittert GA, Tomlinson L, Galletly C, et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab 2004; 89:3337-3344.
61.Wasko R, Komarowska H, Warenik-Szymankiewicz A, Sowinski J. Elevated ghrelin plasma levels in patients with polycystic ovary syndrome. Horm Metab Res 2004; 36:170-173.
62.Orio F Jr, Lucidi P, Palomba S, Tauchmanova L, Cascella T, Russo T, e Circulating ghrelin concentrations in the polycystic ovary syndrome; J Clin Endocrinol Metab 2003; 88:942-945.
63.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002; 87:240-244.
64.Sharma AM. Adipose Tissue: A mediator of cardiovascular disease. Int J Obes 2002; 26:5-7.
65.Felber JP, Golay A. Pathways from obesity to diabetes. Int J Obes 2002; 26:39-45.
66.Ahima RS. Revisiting leptin’s role in obesity and weight loss. J Clin Invest 2008; 118:2380-2383.
67.Iria N, Sonia F, David K, Rocio V, Lucia G. Insulin resistance associated to obesity: The link TNF-alpha. J Clin Endocrinol Metab 2008; 114:183-194.
68.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, et al. Retinol-binding protein 4 in human obesity. Diabetes 55:2805-10.
69.Kusminski CM, Mcternan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clinical Science 2005; 109:243-256.
70.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808.
71.Ryan A, Nicklas B. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care 2004; 27:1699-1705.