Effect of 10% Phenylephrine Eye Drops on Systemic Blood Pressure in Normotensive & Hypertensive Patient
Jagdish Bhatia,1 Mathew Varghese,2 Arti Bhatia 3
ABSTRACT
Objectives: A prospective study to evaluate the effect of 10% Phenylephrine eye drops on systemic blood pressure in normotensive and hypertensive patients.
Methods: The sample comprised of 55 normotensive patients and 34 hypertensive patients were subjected to 10% Phenylephrine eye drops during routine eye examination.
Results: No statistically and clinically significant increase in blood pressure after the instillation of 10% Phenylephrine eye drops was seen in 87% of normotensive patients and 76% of hypertensive patients. Mild rise of blood pressure was seen in 11% of normotensive patients and in 15% of the hypertensive patients. Only one patient (3%) had severe rise of blood pressure in the hypertensive group.
Conclusion: This study shows that pupillary dilatation with 10% Phenylephrine eye drops did not significantly increase systemic blood pressure in normotensive and hypertensive patients. Although precautions should be taken when used in hypertensive patients.
From the Department of Ophthalmology, PO Box: 421, Postal Code: 329, Rustaq, Sultanate of Oman.
Received: 20 Aug 2008
Accepted: 18 Nov 2008
Address correspondence and reprint request to: Dr. Jagdish Bhatia, Department of Ophthalmology, PO Box: 421, Postal Code: 329, Rustaq, Sultanate of Oman.
E-mail: imbahatia@gmail.com.
INTRODUCTION
Pupil dilatation is considered a routine part of a complete eye examination. 10% Phenylephrine eye drops are very frequently used drops in Ophthalmology for dilatation of the pupils especially for fundus examination, for breaking posterior synechiae and before cataract surgery. It is a sympathomimetic drug related to Epinephrine, but is much more stable and produces a more lasting response. It has no effect on the ciliary muscle, so that mydriasis is achieved without any cycloplegia. When instilled into the conjunctival sac, it causes powerful mydriasis, in addition to vasoconstriction.
Systemic complications of topical phenylephrine applied to the eye are those common to sympathomimetics. Phenylephrine 10% eye drops lead to a faster and more pronounced mydriasis but cardiovascular effects such as hypertension and arrhythmias have been reported. In a young healthy adult the upper limit of safety for intravenous administration of phenylephrine is 1.5 mg 1 and Kumar et al 2 had found Phenylephrine plasma levels after administration of topical 10% viscous solution to their patients to be 1.842–11.526 ng/ml after 20 minutes. They concluded that the mean pressure tends to be higher with the 10% viscous solution.
It is usually considered to be safe, but certain reports have appeared in the literature suggesting definite side effects such as acute episodes of systemic hypertension after topical use of 10% phenylephrine hydrochloride.3 British National Formulary recommends caution in use of 10% phenylephrine eye drops, particularly in elderly patients and those with hypertension.4
Chin et al5 in their study on 89 patients concluded that significant hypertensive effects can arise after topical phenylephrine. Symons et al6 reported no significant change in the mean systolic and diastolic blood pressure in 126 patients receiving 10% phenylephrine. Malhotra et al7 in their study on 54 cases showed no difference in systemic cardiovascular effects of either the 2.5% or the 10% concentration. Samantary and Thomas (1975)8 reported a definite increase in blood pressure after topical use of phenylephrine in all of their cases.
In view of these conflicting reports of systemic effects from topical use of 10% phenylephrine, we decided to assess the effect of phenylephrine 10% eye drops on systemic blood pressure in normotensive and hypertensive patients.
METHODS
We carried out a prospective study on 55 normotensive and 34 hypertensive patients undergoing mydriasis in out patient department of our hospital. Those patients who had blood pressure reading of more than 180/100 mm Hg and cardiovascular diseases were not included in the study.
One drop of 10% phenylephrine was instilled into the conjunctival sac of both eyes at 10 minutes interval (twice). Base line blood pressure was recorded before instillation of 10% phenylephrine eye drops. A second reading was taken 60 minutes after instillation. To avoid bias, baseline blood pressure was recorded by one of the co-authors and 60 minutes after instillation, blood pressure was recorded by the other co-author. Both the results were kept secret till the end of the study. To avoid instrumental error, the same manual sphygmomanometer was used for all patients.
RESULTS
Age in the normotensive group varied from 26 years to 75 years (Mean: 50.59 years); and 29 years to 66 years (Mean: 55.66 years) in the hypertensive group. In the normotensive group we had 31 male patients and 24 female patients. While in the hypertensive group we had 10 male patients and 24 female patients. (Figure. 1)
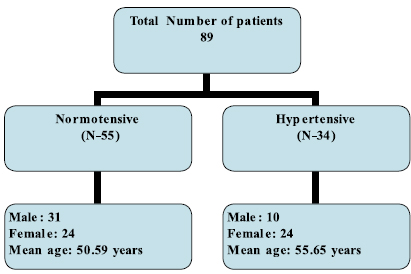
Figure 1: Demographic data of the study
In the normotensive group mean blood pressure before the instillation of 10% phenylephrine eye drops recorded was 130.58/80 mm Hg (SD: 13.33/9.15) which increased marginally to 133.11/82.54 mm Hg (SD: 11.70/7.84) after instillation of Phenylephrine eye drops. Base line mean blood pressure in this group was recorded at 105.18 (SD: 9.57) which increased marginally to 107.79 (SD: 7.59). (Figure 2)
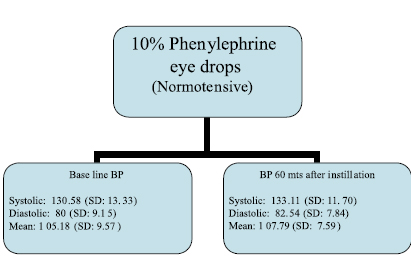
Figure 2: Clinical data of the study (Normotensive group)
In the hypertensive group, mean blood pressure before the instillation of 10% phenylephrine eye drops recorded was 146/87 mm Hg (SD: 20.65/12.85) which increased marginally to 149.95/87.94 mm Hg (SD: 19.03/11.57) after instillation of Phenylephrine eye drops. Baseline mean blood pressure in this group was recorded at 116.53 (SD: 14.99) which increased marginally to 120.59 (SD: 13.68) (Figure 3).
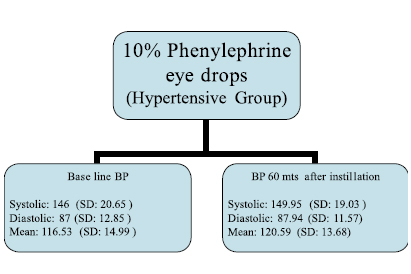
Figure 3: Clinical data of the study (Hypertensive group)
In the normotensive group, we noticed a marginal drop of blood pressure in one patient, while in the hypertensive group marginal drop was seen in two patients. Patients were divided into four groups within each group, depending upon their response to phenylephrine eye drops on blood pressure. Group 1 (No change in blood pressure): Where pre instillation and post instillation variation was 5 mm Hg or less. Group 2 (Mild increase in blood pressure): Where pre instillation and post instillation variation was 6 to 15 mm Hg. Group 3 (Moderate increase in blood pressure): Where difference in pre instillation and post instillation readings was 16 to 30 mm Hg. Group 4 (Severe rise in blood pressure): Where difference in pre instillation and post instillation readings was 31 mm Hg or more.
According to above grouping, 48 of 55 patients in the normotensive group were in group 1 (87%), Group 2 had six patients (11%), Group 3 and Group 4 had no patients. One patient recorded slight decrease in blood pressure after instillation of phenylephrine eye drops (Figure 4).
In the hypertensive group, 26 out of 34 patients were in group 1 (76%), Group 2 had three patients (9%), Group 3 had two patients (6%) and group 4 had one patient (3%). Two patients recorded slight decrease in blood pressure after instillation of phenylephrine eye drops (Figure 4). The only patient in Group 4 in the hypertensive group recorded 34 / 12 mm Hg increase in blood pressure, which was the highest blood pressure increase after instillation of phenylephrine eye drops in the whole study (Figure. 4).
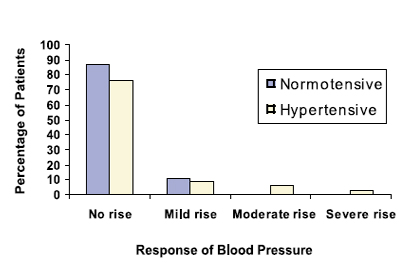
Figure 4: Bar diagram showing percentile response of study group on blood pressure after instillation of 10% phenylephrine eye drops.
DISCUSSION
Systemic reactions to topical use of phenylephrine have been described by various authors. McReynolds et al 9 found no rise in blood pressure in 94 cases and a rise of not more than 10 mm Hg in the remaining six patients (out of 100 hypertensive patients).
Wilensky and Woodward3 described three cases of acute episode of systemic hypertension after instillation of 10% phenylephrine eye drops in the conjunctival sac.
Samantary and Thomas8 studied the systemic effects of topical phenylephrine in 30 normal and 30 hypertensive patients and found a definite increase in blood pressure (both systolic and diastolic) in all the 60 patients after local use of the drug.
Our observations partly support those of McReynolds et al 9 but not those of Samantary and Thomas.8
We found no change in blood pressure in 48 of the normotensive cases (87%) and 26 of the hypertensive cases (76%). Maximum rise of blood pressure in our study was 34/12 mm Hg in one case, more than observed by McReynolds et al in their cases.
We also found a marginal fall in blood pressure in one case from the normotensive group and in two cases from the hypertensive group after instillation of phenylephrine eye drops.
Because of these variable results observed by us, we are inclined to agree with Wilensky and Woodward 3 that in rare cases the instillation of 10% phenylephrine Hcl into the conjunctival sac may be followed by a rise in blood pressure, but it is not possible to say whether rise in blood pressure represents overdose or an individual idiosyncrasy unrelated to dosage of the phenylephrine eye drops when instilled into the conjunctival sac. Our observations are also different from those of Samantary and Thomas,8 who observed a rise in blood pressure in all of their 60 cases, while we found a mild rise in blood pressure in 11% of the normotensive cases and only 18% of the hypertensive cases. But we have not been able to explain fall in blood pressure observed in very few cases in both normotensive (1 patient) and hypertensive groups (2 patients). Unlike McReynolds et al.9 and Wilensky and Woodward 3 but like Samantary and Thomas,8 we did not find any untoward side effects such as palpitation, sweating, trembling and fainting attacks.
CONCLUSION
Eighty nine (89) patients (55 normotensive and 34 hypertensive patients) were studied for the effects of instillation of 10% phenylephrine into the conjunctival sac on systemic blood pressure. It was concluded that while there was a mild to moderate rise in blood pressure in very few patients only, a large percentage of patients from both groups showed no effect on blood pressure. No untoward side effects were observed by us in any patient.
Thus we conclude that a rise in blood pressure followed by instillation into the conjunctival sac of phenylephrine 10% is rare and may be observed in certain individuals; but whether that is due to overdose or individual idiosyncrasy unrelated to dosage or some other factor is not clear; and we are of the view that it should be used in all cases where indicated, keeping in mind the possibility of an idiosyncratic reaction causing rise in systemic blood pressure.
ACKNOWLEDGMENTS
The authors report no conflict of interest and no funding has been received in this work.
-
Fraunfelder FT, Scafidi AF. Possible adverse effects from topical ocular 10% phenylephrine. Am J Ophthalmol 1978; 85:447-453.
-
Kumar V, Schoenwald Rd, Chien DS, Packer AJ, Choi WW. Systemic absorption and cardiovascular effects of phenylephrine eye drops. Am J Ophthalmol 1985; 99:180-184.
-
Wilensky JT, Woodward HJ. Acute systemic hypertension after conjunctival instillation of phenylephrine hydrochloride.Am J Ophthalmol 1973; 76:156-157.
-
British National Formulary. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 1994:389-390.
-
Chin KW, Law NM, Chin MK. Phenylephrine drops in ophthalmic surgery: a clinical study on cardiovascular effects. Med J Malaysia 1994; 49:158-163.
-
Symons RCA, Walland MJ, Kaufman DV. Letter to the editor. Eye 1997; 11:946-947.
-
Malhotra R, Banerjee G, Brampton W, Price NC. Comparison of the cardiovascular effects of 2.5% phenylephrine and 10% phenylephrine during ophthalmic surgery. Eye 1998; 12:973-975.
-
Samantaray S, Thomas A. Systemic effects of topical phenylephrine. Indian J Ophthalmol 1975; 23:16-17
-
McReynolds WU, Havener WH, Henderson JW. Hazards of use of sympathomimetic drugs in ophthalmology. Arch Ophthalmol 1956; 56:176-179.