Serum Uric Acid in Smokers
Bassam E. Hanna, Jamal M. Hamed, Luma M. Touhala
ABSTRACT
Objectives: To demonstrate the possible effect of smoking on serum uric acid.
Methods: Subjects enrolled in study were divided into two groups; nonsmokers and smokers, each with 60 male volunteers of the same social class and dietary habit without history of alcohol consumption, diabetes mellitus, hyperuricemia and gout, renal, joint, lung or heart diseases. Fasting blood and random urine samples were obtained from both groups for measurement of uric acid and creatinine. Calculation of both urine uric acid/urine creatinine ratio and fraction excretion of uric acid were done. The results were statistically evaluated by standard statistical methods.
Results: No significant differences in the age, serum creatinine, spot urine uric acid/urine creatinine ratio and fraction excretion of uric acid between the two groups, serum uric acid was significantly lower in smokers. In smokers there was significant negative correlation of smoking status (average number of cigarette smoked/day, duration of smoking and cumulative amount of smoking) with serum uric acid.
Conclusion: After exclusion of other factors affecting uric acid level, the significant low serum uric acid level in smokers was attributed to reduce endogenous production as a result of chronic exposure to cigarette smoke that is a significant source of oxidative stress. As this reduction is proportionate with smoking status and predisposes to cardiovascular disease, it is, therefore, recommended for smokers to stop or reduce smoking and introduce serum uric acid estimation as routine test since its cheap and simple to reflect their antioxidant level.
Keywords: Smokers; Uric acid; CVD.
Submitted: 25 July 2008
Reviewed: 12 Aug 2008
Accepted: 19 Sept 2008
From the Department of Medicine, Nineveh college of medicine Biochemistry, Mosul, IRAQ
Address Corresponding and reprint request to: Dr. Bassam E. Hanna, Department of Medicine, Nineveh college of medicine Biochemistry, Mosul, IRAQ
Email: bassamhanna2001@yahoo.com
INTRODUCTION
Cigarette-smoking is a well-known risk factor for atherosclerosis development and its complications including cerebral and cardiovascular diseases(CVD)1,2 through vascular endothelial damage3 that possibly occurs through oxygen free radicals production as superoxide radicals, hydrogen peroxide and hydroxyl radicals.4,5 Several enzymes capable of producing oxygen free radicals including xanthine oxidase, NADPH oxidase, myeloperoxidase, and endotoxin.4,5
As cigarette smoke contains superoxide and reactive nitrogen species that readily react with various biomolecules,1,6-9 it has been hypothesized that some of the adverse effects of smoking may result from oxidative damage to endothelial cells, which results in nitric oxide (NO) shortage.1,10,11 (NO) shortage regulates vascular tone that accelerates insufficiency of coronary artery and vasoconstriction in many different tissues.3,12 Therefore imbalance between oxidants and antioxidants may play an important role in the susceptible smoker.13,14 In addition cigarette smokers have increased inflammatory responses that further enhance their oxidative stress.8,9
Since in humans, uric acid is the most abundant aqueous antioxidant, accounting for up to 60% of serum free radical scavenging capacity15 and is an important intracellular free radical scavenger during metabolic stress including smoking,16,17 therefore , measurement of its serum level reflects the antioxidant capacity.15
The aim of this study is to demonstrate the possible effect of smoking on serum uric acid concentration.
METHODS
The study was conducted during the period from March to June 2008, the subjects enrolled in this study were divided into two groups (group I and group II).
Group I, considered as a control group, was composed of 60 apparently healthy nonsmoker male volunteers, their ages ranged from 20 to 69 years.
Group II, considered as a smoker group composed of 60 cigarette-smoker male volunteers, their ages ranged from 16 to 71 years.
A complete record of history was obtained, including name, age, average number of daily cigarettes smoked daily, duration of smoking, dietary habit, alcohol consumption , social class, past medical and drug history.
Members of both groups were within the same social class and dietary habit with no history of alcohol consumption, diabetes mellitus, hyperuricemia and gout, renal, joint, lung or heart diseases.
Fasting blood and random urine samples were obtained from both groups for colorimetric measurement of uric acid and creatinine that were done by Jaffe’s reaction kinetic method18 using a kit supplied by Biolabo Company and by uricase method18 using a kit supplied by Biomeriux Company respectively by obtaining absorbencies using Cecil spectrophotometer-CE 1011 at the Biochemistry Laboratory of Nineveh College of Medicine in Mosul, Iraq.
From the data of serum and urine uric acid and creatinine the calculation of both urine uric acid/urine creatinine (Uuric acid/Ucreatinine) ratio19 and fraction excretion of uric acid (FEuric acid)19,20 were done in both groups, calculation of FEuric acid was done by the following formula(19,20):
FEuric acid = (Uuric acid*Screatinine/Suric acid* Ucreatinine)*100
The results were statistically evaluated by standard statistical methods including mean, standard deviation (SD) range
(minimum-maximum), Linear regression analysis (Pearson correlation coefficient r), student’s t-test21,22 with computer software programs including Microsoft Excel 2003 and SPSS 11.5(23) to evaluate the relation between different parameters of both groups. Differences between observations were considered not significant at p > 0.05.
RESULTS
No statistical significant differences were noted in the age, serum creatinine, spot Uuric acid/Ucreatinine ratio and FEuric acid between two groups (P>0.05, Table I), whereas serum uric acid was significantly lower in group II than group I (P<0.001, Table 1, Fig.1).
The coefficient of variations of serum uric acid and creatinine were 17.68 and 18.46 in group I and 32.14 and 32.46 in group II respectively.
Table 2 demonstrates the mean ± SD and the range of smoking status in group II including the average number of cigarettes smoked/day, duration of smoking and cumulative amount of smoking (calculated by multiplying the number of cigarettes smoked/day with the duration of smoking).
In group II, a statistical significant negative correlations were noted between smoking status parameters and serum uric acid (P<0.001, Fig. 2, Fig. 3, Fig. 4), no such correlations with serum creatinine (P>0.050).
Table 1: Comparison between parameters of both groups; Values are presented as mean ± SD
able 1: Comparison between parameters of both groups; Values are presented as mean ± SD
Parameter |
Group I (Control) n = 60 |
Group II (Smokers) n = 60 |
P-Value |
Age (years) |
33.50 ± 11.80 |
36.10 ± 12.00 |
>0.05 |
Serum creatinine (µmol/L) |
96.82 + 17.87 |
89.84 + 29.16 |
>0.05 |
Serum uric acid (mmol/L) |
0.27+ 0.05 |
0.22 + 0.07 |
P<0.001 |
Uuric acid/Ucrearitine |
0.43 + 0.11 |
0.41 + 0.17 |
>0.05 |
FEuric acid (%) |
10.21 + 2.38 |
11.85 + 6.16 |
>0.05 |
Table 2: Smoking status in group II; Values are presented as mean ± SD
Parameter |
Mean ± SD |
Range |
Number of cigarette/day |
26.75 + 16.41 |
5 - 80 |
Duration of smoking (Years) |
12.75 + 8.86 |
1.5 -35 |
Cumulative amount of smoking |
432.63 + 499.58 |
7.5 - 2100 |

Figure 1: Spread of uric acid levels in group I and group II

Figure 2: Correlation between serum uric acid and number of cigarette smoked/day in group II
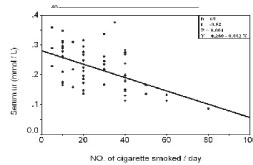
Figure 3. Correlation between serum uric acid and duration of smoking in group II
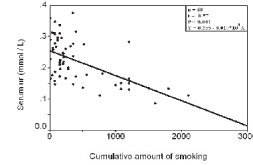
Figure 4. Correlation between serum uric acid and cumulative amount of smoking in group II
DISCUSSION
Many but not all epidemiological studies have suggested that high serum uric acid is a risk factor for CVD24-28 and warranted to evaluate its prognostic implications and potential utility in the monitoring of therapy.29 This raised level of serum uric acid parallel to an increased risk of CVD could be either primary or secondary to underlying causes of CVD.30-35 However, the specific role of serum uric acid in this constellation remains uncertain36 although may be involved in platelet adhesiveness, aggregation or inflammation37-39 and may be implicated in the genesis of hypertension.40
In contrast , there is some evidence suggesting that the increase of serum uric acid is protective against CVD since uric acid acts as an endogenous antioxidant41-43 and the higher serum uric acid levels found in CVD patients suggests that any protective antioxidant effect which uric acid has is overwhelmed by other negative effects on pathogenesis.44 Recently the viability of administering uric acid in solution has been established41even they do not specifically address the question of a biological link between uric acid and mechanisms of endothelial dysfunction or atherosclerosis since inhibition of xanthine oxidase by allopurinol is likely to reduce the production of hydrogen peroxide and thereby ameliorate oxidative stress even in smokers45 independent of its effects on uric acid.46 Furthermore, allopurinol has antioxidant properties.47 Therefore, raising serum uric acid concentrations protects against acute oxidative damage as in smoking.48
In this study, although serum uric acid level in smokers did not reach the lower reference range (0.12 mmol/L)49 however it is significantly lower than the nonsmoker group (P<0.001) and has significant negative correlation with smoking status including the average number of cigarettes smoked/day, duration of smoking and cumulative amount of smoking. This finding is in agreement with other studies that showed low serum uric acid in regular smokers1,50 and reduction of antioxidants including uric acid in smokers51,52 indicating that oxidative stress increases everytime a cigarette is smoked.1 Other studies proved that even nonsmokers exposed to cigarette smoke have a significantly lower plasma antioxidant status than unexposed nonsmokers do, independent of differences in dietary antioxidant intakes.51 It even proved that administration of uric acid raises circulating antioxidant defenses and allows restoration of endothelium-dependent vasodilation.11,52 Therefore, high serum uric acid concentrations might be protective in situations characterized by increased cardiovascular risk and oxidative stress as smoking,11 and by reducing its level it increases susceptibility to oxidative damage and accounts for the excessive free radical production.53 Therefore, the possibility that uric acid confers protection against the development of atherosclerosis, in view of its antioxidant properties, has been recognized.54,55
In this study, serum creatinine, FEuric acid and Uuric acid/Ucreatinine ratio are not significantly differ between two groups, in addition to that FEuric acid and Uuric acid/Ucreatinine ratio values lie within the mean ± SD observed in the control group by other studies.19,56-58 Since these tests have been reported to be useful to evaluate renal handling of uric acid59-63 and as serum uric acid concentrations are highly dependent on endogenous production as well as renal excretion.64,65 Therefore, low serum uric acid level in smokers is attributed to reduction of endogenous production. As result of smoking, oxidative stress consumption rather than increased renal excretion after exclusion of other factors that affect its level as all members of groups I and II are subjected to the same preanalytical and postanalytical factors and all of them are within the same social class, dietary habit with no history of alcohol consumption in addition to standardization of laboratory uric acid measurement to avoid analytical variations.
This finding is in agreement with other studies that proved that reduction of antioxidants including uric acid in smokers66 is due to both chronic exposure to cigarette smoke that is a significant source of oxidative stress8,53 and low intake of dietary antioxidants.67
CONCLUSION
After exclusion of other factors affecting uric acid level, the significant low serum uric acid level in smokers was attributed to reduce endogenous production as a result of chronic exposure to cigarette smoke that is a significant source of oxidative stress. As this reduction is proportionate with smoking status and predisposes to cardiovascular disease, it is recommended for smokers to stop or reduce smoking and introduce serum uric acid estimation as routine test since its cheap and simple to reflect their antioxidant level.
REFERENCES
-
Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma .Circulation 2002 ; 105: 1155-1157.
-
Celermajer DS, Sorensen K, Georgakopoulis D, Bull C, Thomas O, Robinson J, et al. Cigarette-smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilatation in healthy young adults. Circulation 1993; 88: 2149–2155.
-
Benzuly KH, Padgett RC, Kaul S, Piegors DJ, Armstrong ML, Heistad DD. Functional improvement precedes structural regression of atherosclerosis. Circulation 1994; 89:1810–1818.
-
Calver A, Collier J, Moncada S, Vallance P. Effect of intraarterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide mechanism appears abnormal. J Hypertens 1992; 10:1025–1031.
-
Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 1999; 100:1161–1168.
-
Karnath B. Smoking cessation. Am J Med 2002; 112: 399-405.
-
Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 1997; 105(suppl): 875-882.
-
Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology 2002; 180: 121-137.
-
Brown KM, Morrice PC, Duthie GG. Erythrocyte vitamin E and plasma ascorbate concentrations in relation to erythrocyte peroxidation in smokers and nonsmokers: dose response to vitamin E supplementation. Am J Clin Nutr 1997; 65: 496-502.
-
Node K, Kitakaze M, Yoshikawa H, Kosaka H, Hori M. Reversible reduction in plasma concentration of nitric oxide induced by cigarette smoking in young adults. Am J Cardiol 1997; 79:1538–1541.
-
Waring SW, McKnight JA, Webb DJ, Maxwell SRJ. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 2006; 55: 3127-3132.
-
Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health 1989; 79:158–162.
-
Van der Vaart H, Postma DS, Timens W, Ten Hacken NHT. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 2004; 59: 713-721.
-
Polidori MC, Mecocci P, Stahl W, Sies H. Cigarette-smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. Br J Nutr 2003; 90:147-150.
-
Maxwell SR, Thomason H, Sandler D, LeGuen C, Baxter MA, Thorpe GH, et al. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1997; 27: 484–490.
-
Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney MW. Tourniquet-induced xsanguinations in patients requiring lower limb surgery: an ischemia-reperfusion model of oxidant and antioxidant metabolism. Anesthesiology 1996; 84:14 –22.
-
Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med 1997; 22:169–174.
-
Whelton A, Watson JA, Robert RC. Nitrogen metabolism and renal function. In: Tietz fundamentals of clinical chemistry (Burtis CA, Ashwood ER, eds). 4th ed. Saunders com 1996; 578-580.
-
Perez-Ruiz F, Calabozo M, García-Erauskin G, Ruibal A, Herrero Beites AM. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 2002; 47:610–613.
-
Masayoshi S, Yukio H. Serum uric acid level and fractional excretion of urate in fluid and electrolyte disturbances. Japanese Journal of Clinical Pathology 1999. 47:417-423.
-
Chap TLE. Introductory biostatistics. Wiely interscience 2003; 283 – 292.
-
Jones D. Pharmaceutical statistics. Pharmaceutical press 2002.
-
Landau S, Everitt BS. A Handbook of Statistical Analyses using SPSS. A CRC Press Company USA 2003.
-
Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of serum uric acid to mortality and ischemic heart disease. Am J Epidemiol 1995; 141: 637-644.
-
Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death . Ann Intern Med 1999; 131: 7-13.
-
Rich MW. Uric acid: is it a risk factor for cardiovascular disease? Am J Cardiol 2000; 85:1018–1021.
-
Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000; 283: 2404–2410.
-
Meisinger C, Koenig W, Baumert J, Döring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol 2008; 28: 1186-1192.
-
Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008; 58: 623-630.
-
Whitelaw DC, O’Kane M, Wales JK, Barth JH. Risk factors for coronary heart disease in obese non-diabetic subjects. Int J Obes Relat Metab Disord 2001; 25: 1042–1046.
-
Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003; 41: 1183–1190.
-
Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension 2005; 45:18–20.
-
Wheeler JG, Juzwishin KD, Eiriksdottir G, Gudnason V, Danesh J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med 2005; 2: e76.
-
Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J 2006; 27: 1174 –1181.
-
Kawamoto R, Tomita H, Oka Y, Ohtsuka N. Relationship between serum uric acid concentration, metabolic syndrome and carotid atherosclerosis . Intern Med 2006; 45: 605– 614.
-
Dzielak DJ, Kivlighn SD. Emerging concepts in cardiovascular disease. Exp Opin Invest Drugs 1998; 7: 85-89.
-
Mustard JF, Murphy EA, Ogryzlo MA. Blood coagulation and platelet economy in subjects with primary gout. CMAJ 1963; 89: 1207-1211.
-
Newland H. Hyperuricemia in coronary, cerebral, and peripheral arterial disease: an explanation. Med Hypotheses 1975; 1: 152-155.
-
Selby JV, Friedman GD, Quesenberry CP Jr. Precursors of essential hypertension. Am J Epidemiol. 1990; 131: 1017-1027.
-
Nicholls A, Snaith ML, Scott JT. Effect of estrogen therapy on plasma and urinary levels of uric acid. BMJ 1973; 1: 449-451.
-
Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 2001; 38: 365–371.
-
Maxwell AJ, Bruinsma KA. Uric acid is closely linked to vascular nitric oxide activity. Evidence for mechanism of association with cardiovascular disease. J Am Coll Cardiol 2001; 38: 1850–1858.
-
Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Barry G, Pearlson GD. Serum Uric Acid and Cognitive Function in Community-Dwelling Older Adults. Neuropsychology 2007; 21: 136–140.
-
Torun M, Yardım S, Simsek B, Burgaz S. Serum uric acid levels in cardiovascular diseases . J clinical pharmacy and therapeutics 2008; 23: 25-29.
-
Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine Oxidase Inhibition Reverses Endothelial Dysfunction in Heavy Smokers. American Heart Association Clinical Investigation and Reports. Circulation 2003; 107: 416.
-
Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 2002; 51: 1118 –1124.
-
Ricardo SD, Bertram JF, Ryan GB. Podocyte architecture in puromycin aminoglycoside-treated rats administered tungsten or allopurinol. Exp Nephrol 1995; 3:270 –279.
-
Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci 2003; 105: 425– 430.
-
Boon NA, Colledqe NR, Walker BR, Hunter J. Davidson’s principle and practice of medicine. Churchill livinqstone 2006: 1320.
-
Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 1998; 31:552–557.
-
Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr 2003; 77:160-166.
-
Goraca A, Skibska B. Plasma antioxidant status in healthy smoking and non-smoking men. Bratisl L Listy 2005; 106:301-306.
-
Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 1996; 94:19 –25.
-
Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med 1993; 14: 615– 631.
-
Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis 2000; 148: 131–139.
-
Pak CY. Renal Calculi. In: Cecil Textbook of Medicine, Volume 1 (Wyngaarden JB and Smith LH Jr). 18th ed. Philadelphia PA: WB Saunders com 988; 638-644.
-
Pesce AJ, Kaplan LA. Methods in Clinical Chemistry, St Louis, MO: Mosby-Year Book Inc, 1987, 32.
-
Katopodis KP, Elisaf MS, Pappas HA, Theodorou JCh, Milionis HJ, Bourantas KL, et al. Renal abnormalities in patients with sickle cell-beta thalassemia. J of Nephrology 1997; 10: 163-167.
-
Puig JG, Mateos FA, Jimenez ML, Ramos TH. Renal excretion of hypoxanthine and xanthine in primary gout. Am J Med 1988; 85: 533–537.
-
Nishida Y, Ito K. Decreased renal phosphate threshold in patients with gout. Nephron 1992; 62:142–144.
-
Emmerson BT. The management of gout. N Engl J Med 1996; 334: 445–451.
-
Tykarsky A. Evaluation of renal handling of uric acid in essential hypertension: hyperuricemia related to decreased urate secretion. Nephron 1991; 59: 364–368.
-
Perez-Ruiz F, Calabozo M, Herrero A, Ruiz-Lucea E, Alonso Ruiz A. Analysis of the methods for classifying gout according to renal excretion of uric acid. Rev Esp Reumatol 1998; 25: 335–339.
-
Leyva F, Wingrove CS, Godsland IF, Stevenson JC. The glycolytic pathway to coronary heart disease: a hypothesis. Metabolism 1998; 47: 657–662.
-
Quinones GA, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995; 268: E1–5.
-
Tomita M, Mizuno S, Yokota K. Increased levels of serum uric acid among ex-smokers. J Epidemiol 2008; 18: 132-134.
-
Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr J 2007; 6:39.