Acute myeloid leukemia (AML) is a clonal over-proliferation of stem cells that fail to differentiate, associated with accumulation of non-functional myeloid cells (myeloblasts). In spite of advanced therapeutic options, a considerable number of AML patients still relapse, even those with favorable or intermediate risk profiles.1
Several prognostic markers including molecular ones have been studied in AML to provide prognostic stratification of patients and identify those with potentially poor outcomes who may require more investigational trials and aggressive therapy.2 Cytogenetic analysis of leukemic blasts has emerged as an important foreteller of overall survival and remission.3
The cluster of differentiation 38 (CD38) protein is an important marker in hematologic malignancies, mainly multiple myeloma and chronic lymphocytic leukemia.4,5 It is a transmembrane glycoprotein with multi-functional properties, mainly in adhesion, migration, and signal transduction. CD38 is a partner for the adhesion of CD31 and is involved as the ectoenzyme in the catabolism of nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate.6,7 In the hematopoietic system, CD38 is expressed by lymphoid cells, myeloid cells, platelets, erythrocytes, and plasma cells that depict maximum expression.7 Its maximum expression on plasma cells compared to other cells makes it a perfect target for therapy in multiple myeloma.8
Despite increasing knowledge about the molecular and biochemical functions of CD38, a controversy persists regarding its clinical significance in adult AML. Therefore, we studied CD38 expression in relation to the standard cytogenetic risk stratification in AML patients to assess its relation to their prognoses.
Methods
The design of this study was prospective. The subjects were 52 patients newly diagnosed with AML during the period January 2017 to December 2018, at the Hematology Department of Alexandria Main Hospital and Medical Research Institute, Alexandria, Egypt.
The study included newly diagnosed AML patients between 18 and 64 years of age. Excluded patients were ones with mixed lineage expression acute leukemia and AML patients with the following characteristics: having autoimmune diseases and coexisting secondary malignancy. We also excluded AML patients aged ≥ 65 years to preempt the confounding effect of aging on CD38 expression.
This study was part of a study on AML patients, approved by the ethical committee of the Faculty of Medicine, Alexandria University, Egypt [IRB No. 00007555 - FWA No. 00015712]. Informed consent was obtained from patients before they were included in this study.
The diagnosis of AML was established by the presence of bone marrow blasts of ≥ 20% and the characteristic flowcytometry of myeloid blasts using monoclonal antibodies of acute panel labeled by red color phycoerythrin or green color fluorescein isothiocyanate. The CD38 expression was analyzed simultaneously with the same run of flow cytometry for each patient. Positive CD38 expression was defined by the presence of ≥ 20% of myeloblasts expressing the antigen. The control group comprised of 25 normal bone marrow samples (age-and-sex-matched) extracted during the same period in our lab.
Cytogenetic analyses were conducted by fluorescence in situ hybridization technique in addition to molecular studies in some patients. Based on cytogenetic results, the patients were stratified into three groups: ‘favorable risk’, ‘intermediate risk’, and ‘poor risk’.9 Favorable cytogenetic risk included: t(8;21)(q22;q22) regardless to other cytogenetics, inv(16)(p13p22) or t(16;16)(p13;p22), and/or detection of a CBFβ-MYH11 translocation product, regardless of other cytogenetics. The poor cytogenetic group included: monosomy 5, monosomy 7, del (5q), del (7q), inv (3)(q21q26.2), t (3;3)(q21;q26.2), 11q23 aberrations, or complex karyotype (≥ 3 aberrations not involved in the low-risk group). Intermediate cytogenetic risk included all other aberrations that were not considered favorable or high risk.9
Leukemic blasts gating was performed by forward versus side scatter plots using specimens prior to the start of treatment. For specimens of the control group, gating was done around the mononuclear cells (forward versus side scatter plots) [Figure 1].
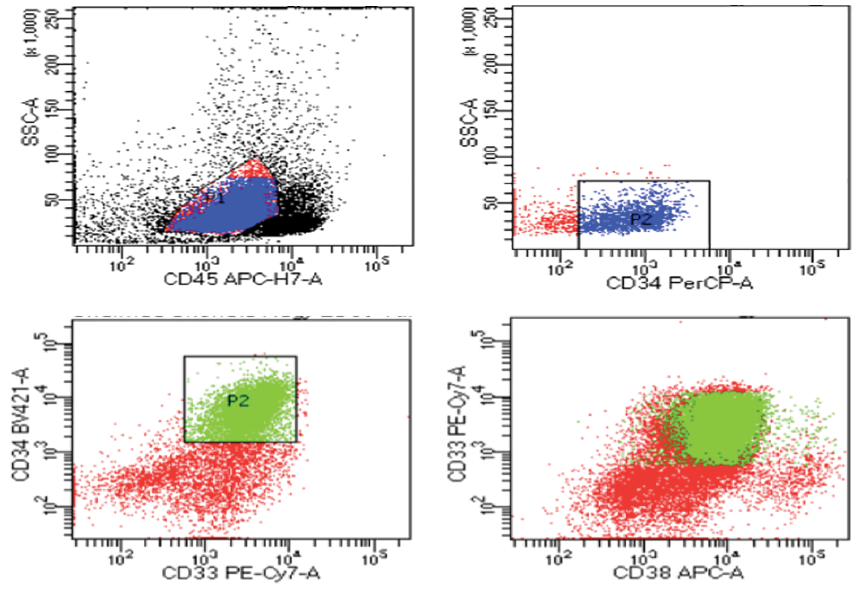 Figure 1: Flow cytometry of one of the cases; gating and sorting of myeloblasts and expression of CD38.
Figure 1: Flow cytometry of one of the cases; gating and sorting of myeloblasts and expression of CD38.
Each patient was followed-up for two years from the date of diagnosis. Kaplan Meier survival analysis was used to present the median overall survival. Data was analyzed using SPSS Statistics (IBM SPSS Statistics for Windows, version 24 ). The quantitative variables were tested for normality distribution, patient age, and hemoglobin concentrations and presented as mean and SDs verified by paired t-tests. Non-parametric statistics were presented as medians and ranges, verified by the Mann-Whitney U test. Qualitative variables were subjected to a chi-square test or Fisher exact test as appropriate. The Kaplan-Meier method was used to describe the median overall survival in regard to CD38 expression as well as to the cytogenetic risk groups and confirmed using the log-rank test.
In all statistical tests, we used the 95% CI and p-value ≤ 0.05 was considered statistically significant.
Results
The age of the studied AML patients (N = 52) ranged from 18 to 64 (mean 43.1±12.5) years. Male patients were the majority (61.5%). The median of hematological parameters showed significantly marked anemia, thrombocytopenia, and leukocytosis. The count of blasts in bone marrow ranged 28.0–95.0% with a median of 61.0%. The percentage of CD38 expression among AML patients ranged 3.6–79.9%, and was positive in 35 (67.3%) patients and negative in 17 (32.7%) patients. The CD38 expression in AML patients was significantly higher than in the control (p = 0.001) [Table 1].
Table 1: Patients’ characteristics, CD38 expression, and some laboratory parameters of the studied patients with acute myeloid leukemia (AML) compared to the control.
|
Sex
|
|
|
|
|
Male
|
32 (61.5%)
|
14 (56.0%)
|
0.643
|
|
Female
|
20 (38.5%)
|
11 (44.0%)
|
|
|
Mean age, years
|
43.1 ± 12.5
|
45.6 ± 12.3
|
0.411
|
|
CD38 expression %, median (range)
|
36.6 (3.6–79.9)
|
12.4 (1.5–31.7)
|
0.001*
|
|
Hemoglobin concentration (g/dL), mean (SD)
|
7.61 ± 2.3
|
14.6 ± 2.4
|
0.001*
|
|
Total leukocytic count (x109/L), median (range)
|
18.5 (1.2–235.0)
|
7.3 (4.1–10.0)
|
0.001*
|
|
Platelets count (x109/L), median (range)
|
42.0 (14.0–140.0)
|
231.0 (180.0–430.0)
|
0.001*
|
* significant.
Regarding clinical symptoms, the majority of our AML patients presented with fatigue and bone pain, while half of them presented with bleeding. On the basis of the French-American-British (FAB) classification, the common diagnostic types found were AML-M2 (30.8%) and AML-M4 (26.9%) [Table 2].
Table 2: Clinical data and FAB classification of the patients with acute myeloid leukemia (AML).
|
Clinical data
|
|
|
|
Fatigue
|
44
|
84.6
|
|
Bone pain
|
35
|
67.3
|
|
Bleeding
|
28
|
53.8
|
|
Fever
|
22
|
42.3
|
|
Hepatomegaly
|
16
|
30.8
|
|
Splenomegaly
|
14
|
26.9
|
|
Lymphadenopathy
|
4
|
7.7
|
|
FAB classification type
|
|
|
|
AML-M1
|
4
|
7.7
|
|
AML-M2
|
16
|
30.8
|
|
AML-M3
|
9
|
17.3
|
|
AML-M4
|
14
|
26.9
|
|
AML-M5
|
6
|
11.5
|
|
AML-M6
|
2
|
3.8
|
FAB: French-American-British. All percentages are based on the total sample size of AML patients (N = 52).
The patients were classified according to cytogenetic findings as favorable cytogenetics (n = 16), intermediate cytogenetics (n = 14), and poor cytogenetics (n = 22). The percentage of AML patients with positive CD38 expression was higher in the group with favorable cytogenetics (75.0%) than in the groups with intermediate (57.1%) and poor cytogenetics (68.2%). However, these differences were not significant (p = 0.578). Meanwhile, the median CD38 expression was significantly higher in favorable cytogenetic group patients (55.0%) than in intermediate (26.2%) and poor (27.9%) cytogenetic groups (p = 0.007) [Table 3].
Table 3: CD38 expression in relation to cytogenetic risk category in acute myeloid leukemia patients.
|
Negative CD38
|
4 (25.0%)
|
6 (42.9%)
|
7 (31.8%)
|
0.578
|
|
Positive CD38
|
12 (75.0%)
|
8 (57.1%)
|
15 (68.2%)
|
|
*significant.
The Kaplan Meier median survival curve for CD38 expression was higher in patients who were CD38+ (20.9 months) compared to those who were CD38– (18.1 months). However, this difference was found insignificant (p = 0.090) [Figure 2].
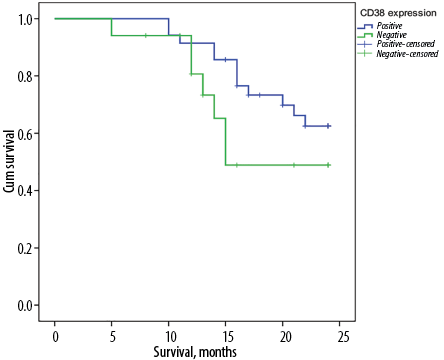 Figure 2: The median overall survival time in relation to CD38 expression in acute myeloid leukemia patients.
Figure 2: The median overall survival time in relation to CD38 expression in acute myeloid leukemia patients.
Among our AML patients with positive CD38 expression, those with poor cytogenetic risk had significantly shorter median overall survival (17.9 months) than those with favorable and intermediate cytogenetic risk (23.5 and 22.6 months, respectively) (p = 0.010) [Figure 3].
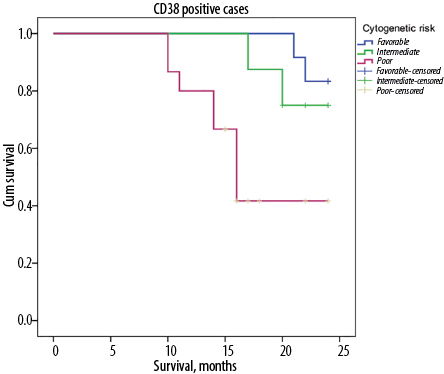 Figure 3: The median overall survival time in relation to cytogenetic risk categories (favorable, intermediate, and poor) among positive CD38 patients with acute myeloid leukemia.
Figure 3: The median overall survival time in relation to cytogenetic risk categories (favorable, intermediate, and poor) among positive CD38 patients with acute myeloid leukemia.
Discussion
CD38 has been thoroughly studied in patients with lymphocytic disorders, mainly chronic lymphocytic leukemia and multiple myeloma.10 In the current study, the expression of CD38 was studied in patients with AML to document the findings in relation to their cytogenetic risk.
As CD38 is widely expressed by inflammatory cells, even low-grade inflammation such as that associated with normal aging can lead to an increased expression of CD38.11 For this reason, we excluded patients aged ≥ 65 years.
CD38 was expressed in variable percentages in all the AML patients in this study. Before the study, we had expected to find an increased expression of CD38 in bone marrow samples. Weekx et al,12 compared the percentage of human hematopoietic progenitor cells that were “CD34+ CD38- and CD34+ CD38+” from three sample sources: adult bone marrow, fetal liver, and cord blood. They observed that a significantly higher percentage of CD34+ cells were CD38+ in adult bone marrow than in fetal liver and cord blood.
Positive expression of CD38 ≥ 20% was detected among 67.5% of them. This expression was found statistically significantly higher in AML patients than in the control. Similarly, Naik et al,13 have reported positive CD38 expression in blast cells to be heterogenous and frequently exceeds that of normal cell populations.
Till now, the risk schemes in AML have been primarily based on cytogenetic risk groups,9 while the current study approach is also based on CD38 expression in addition to this cytogenetic risk. We found that increased CD38 expression was significantly higher in favorable cytogenetic group patients and decreasing in intermediate and poor cytogenetic groups.
In the study of Repp et al,14 among 783 newly diagnosed AML patients at the German SHG-AML trials in 1991 and 1996, the univariate analysis depicted that positive CD38 expression was related to favorable cytogenetics. Our findings are similar. Additionally, a Chinese study with 56 AML patients found that CD38 was variably expressed in all FAB types, cytogenetic risk groups, and on cells from patients who had or had not responded to therapy.15 They concluded that the amount of detectable CD38+ cells could be predictive for the further course of the disease. Moreover, Keyhani et al,16 suggested that increased CD38 expression was associated with a favorable prognosis in adult acute leukemia. However, Naik et al,13 in their study to test CD38 expression on leukemic blasts using flowcytometry of 37 patients with AML and 12 patients with T-acute lymphoblastic leukemia, reported no correlation between CD38 expression and the FAB classification or the European Leukemia Network risk stratification in AML.
The study of Keyhani et al,16 depicted CD38 expression in AML patients as a helpful distinct foreteller of the outcome of the disease. Their patients with higher CD38 expression had significantly longer complete response duration and survival (p = 0.036 and p = 0.048, respectively). Our AML patients with positive CD38 expression also showed higher median overall survival when compared to negative CD38 AML patients. Even when only positive CD38 cases were studied, significantly shorter median overall survival was associated with AML patients having poor cytogenetics. An increased CD38 expression might be used as a differentiation marker and consequently is related to better overall survival.
On the contrary, Dwivedi et al,17 suggested that cells overexpressing CD38 in the tumor microenvironment may lead to immune suppression which reduces the function of effector T cells and encourages angiogenesis, providing an immune evasion that assists in cancer progression.
Conclusion
This study concluded that CD38 expression adds to the prognostic value of cytogenetic risk stratification at diagnosis of AML patients. Further studies are needed to confirm these results. Testing of CD38 expression should be included in the standard panel for diagnosis of AML patients for better evaluation of the prognosis and deciding on treatment options.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015 Sep;373(12):1136-1152.
- 2. Lin TL, Smith BD, Lopez FA. Prognostically important molecular markers in cytogenetically normal acute myeloid leukemia. Am J Med Sci 2011 May;341(5):404-408.
- 3. Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al; Cancer and Leukemia Group B (CALGB 8461). Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from cancer and leukemia group B (CALGB 8461). Blood 2002 Dec;100(13):4325-4336.
- 4. Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F. CD38: a target for immunotherapeutic approaches in multiple myeloma. Front Immunol 2018 Nov;9:2722.
- 5. Burgler S. Role of CD38 expression in diagnosis and pathogenesis of chronic lymphocytic leukemia and its potential as therapeutic target. Crit Rev Immunol 2015;35(5):417-432.
- 6. Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 2006 Jul;345(4):1386-1392.
- 7. Morandi F, Airoldi I, Marimpietri D, Bracci C, Faini AC, Gramignoli R. CD38, a receptor with multifunctional activities: from modulatory functions on regulatory cell subsets and extracellular vesicles, to a target for therapeutic strategies. Cells 2019 Nov;8(12):1527-1543.
- 8. van de Donk NW, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018 Jan;131(1):13-29.
- 9. Montserrat E, Hillmen P. Cytogenetics and molecular risk groups in acute myeloid leukaemia. In: Hoffbrand AV, Higgs DR, Keeling DM, Mehta AB, editors. Postgraduate haematology. 7th ed. UK: Wiley Blackwell; 2016. p. 365.
- 10. Schuh W, Mielenz D, Jäck H-M. Unraveling the mysteries of plasma cells. Adv Immunol 2020;146:57-107.
- 11. Camacho-Pereira J, Tarragó MG, Chini CC, Nin V, Escande C, Warner GM, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab 2016 Jun;23(6):1127-1139.
- 12. Weekx SF, Van Bockstaele DR, Plum J, Moulijn A, Rodrigus I, Lardon F, et al. CD34++ CD38- and CD34+ CD38+ human hematopoietic progenitors from fetal liver, cord blood, and adult bone marrow respond differently to hematopoietic cytokines depending on the ontogenic source. Exp Hematol 1998 Oct;26(11):1034-1042.
- 13. Naik J, Themeli M, de Jong-Korlaar R, Ruiter RW, Poddighe PJ, Yuan H, et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica 2019 Mar;104(3):e100-e103.
- 14. Repp R, Schaekel U, Helm G, Thiede C, Soucek S, Pascheberg U, et al; AML-SHG Study Group. Immunophenotyping is an independent factor for risk stratification in AML. Cytometry B Clin Cytom 2003 May;53(1):11-19.
- 15. Petrovici K, Graf M, Reif S, Hecht K, Schmetzer H. Expression profile of the progenitor cell markers CD34, CD38 and CD90 in acute myeloid leukemia and their prognostic significance. J Cancer Molecules 2010;5(3):79-86.
- 16. Keyhani A, Huh YO, Jendiroba D, Pagliaro L, Cortez J, Pierce S, et al. Increased CD38 expression is associated with favorable prognosis in adult acute leukemia. Leuk Res 2000 Feb;24(2):153-159.
- 17. Dwivedi S, Rendón-Huerta EP, Ortiz-Navarrete V, Montaño LF. CD38 and regulation of the immune response cells in cancer. J Oncol 2021 Feb;2021:6630295.