Superior Vena Cava Syndrome: A Presenting Feature of Mediastinal Germ Cell Tumor
Mahua Roy,1 Rajat Bandyopadhyay,2 Narayan Pandit,3 Soumita Sengupta4
Roy M, et al. OMJ. 25, 131-133 (2010); doi:10.5001/omj.2010.35
ABSTRACT
Superior vena cava syndrome (SVCS) is rare in children. Non-Hodgkin’s Lymphoma (NHL) is the most common cause of SVCS in children. This report an adolescent male who presented with SVCS due to mixed germ cell tumor (GCT) of the anterior mediastinum with predominant yolk cell component. Such etiology of SVCS is rarely reported.
From the 1Department of Pediatric Medicine. North Bengal Medical College & Hospital. Darjeeling,2Department of Radiotherapy, RMO- Cum- Clinical- Tutor. Radiotherapy, North Bengal Medical College & Hospital, Darjeeling, 3Department of Radio-Diagnosis. North Bengal Medical College & Hospital. Darjeeling. 4Department of Pathology. North Bengal Medical College & Hospital, Darjeeling.
Received: 13 Nov 2009
Accepted: 31 Jan 2010
Address correspondence and reprint request to: Dr. Mahua Roy. (MD. Pediatric Medicine), Asst. Prof. Pediatric Medicine. North Bengal Medical College & Hospital. Darjeeling.
E-mail: drmahuaroy@gmail.com
Roy M, et al. OMJ. 25, 131-133 (2010); doi:10.5001/omj.2010.35
INTRODUCTION
The most common cause of SVCS in children is non-Hodgkin lymphoma (NHL) while in adults, it is lung cancer.1 Other than NHL, T-cell acute lymphoblastic leukemia, Hodgkin’s lymphoma and Tubercular mediastenitis can manifest as SVCS in children. This is a report of an adolescent male who presented with SVCS due to mixed germ cell tumor of the anterior mediastinum with predominant yolk cell component. Such etiology of SVCS has rarely been reported in literature.
A 12 year old male was presented with facial puffiness, irregular fever, cough and breathlessness for two weeks. Breathlessness was gradually increased and became distressing from the previous night. There was no history of expectoration, hemoptysis, pedal edema, decreased urine output or contact with tuberculosis.
Clinical examination revealed an anxious, plethoric, dyspnoeic adolescent with facial puffiness. Distended non-pulsatile superficial veins were seen over the temple and neck region. However, cyanosis or peripheral lymphadenopathy was absent. The patient had a respiratory rate of 42/min, heart rate of 138/min, blood pressure of 124/76 mmHg and oxygen saturation (SpO2) of 100% O2 was was 97%. Chest movements were diminished and right hemithorax was bulged with visible venous engorgement. Apex beat and trachea was shifted to the left. Breath sounds were absent on right mid and lower zones and diminished on left lower zone with overlying dullness on percussion. Only a mild hepatomegaly was detected, but there was no splenomegaly, ascites and intra abdominal or testicular mass. Other systemic examinations were unremarkable.
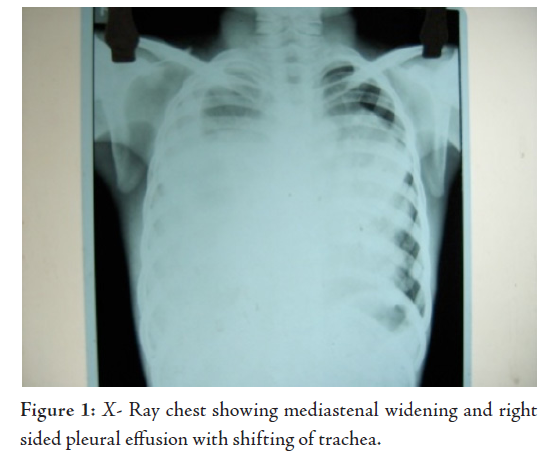
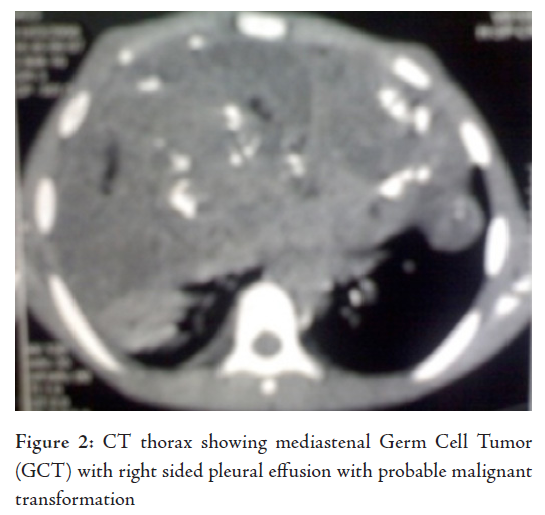
Laborator tests revealed that hemogram, renal function tests and serum electrolytes were all within normal limits. Chest X-ray showed right sided pleural effusion, while the right dome of the diaphragm and right cardiac border was obscured by a heterogenous opacity in the right mid and lower zone. The lower mediastenum was shifted towards the left side and but the left costrophrenic angle was clear, (Fig. 1). Abdominal sonography showed mild hepatomegaly with homogenous echotexture and normal testis but without ascites or lymphadenopathy. Computerized tomography (CT) scan of the thorax (plain and contrast) showed a large heterogeneous poorly defined space-occupying lesion (SOL) in the right hemithorax infiltrating the right lung and mediastinum and occupying a large area of the right hemithorax, causing a mass effect with a shift of trachea and mediastinal structure to the left. Parietal pleura thickened and appear to be merged with the described SOL. There was right sided pleural effusion extending across the anterior mediastinum to the left. The CT scan characters suggested heterogeneous nature with area of calcification and fat density. Visualized parenchyma of the right lung was normal. Hence mediastinal lymphaenopathy was absent. Mild heterogeneous enhancement was seen in IV contrast study. The CT scan feature was suggestive of mediastenal germ cell tumor with possibilities of malignant changes (Fig. 2). The echocardiogram showed presence of anterior mediastinal mass with small pericardial effusion without myocardial involvement, cardiac contractility was not impaired. Serum Alpha-fetoprotein was hugely raised to more than 1210 ng/ml (normal range is less than 8.5). Beta HCG was also very high, it was 26.33 mIu/ml (normal range is less than 2) and alkaline phosphatase was 308 U/L. CT guided fine needle aspiration cytology (FNAC) detected mature squamous cell, mature fat cells, and few inflammatory cells. In some areas, there were loosely cohesive epithelial-like cells having mild pleomorphism, round nuclei and prominent cytoplasmic vaccuolation. Intracytoplasmic hyaline globule-like structures were detected in few of those cells. The CT scan findings along with biochemical parameters and cytological features suggested a mixed germ cell tumor predominantly composed of mature teratoma with areas of yolk sac component.
Emergency management was initiated with elevation of the head and 100% moist O2 inhalation. Diuretics and intravenous bolus dose of methyl prednisolone (30mg/kg/dose for 3 days) was given. As extensive lung involvement was a deterrent factor against a curative surgery, reasonable chemotherapy consisting of the BEP regimen in standard doses was offered (Cisplatin 20mg/m2 d1-d5, Etoposide 100mg/m2 d1-d5, Bleomycin 15 units d1, d8, d15.) There was appreciable response to the first cycle of chemotherapy. Unfortunately, the patient defaulted due to financial constraints, and died after completion of the long delayed second cycle of chemotherapy after six months.
DISCUSSIONSince SVCS was first described by William Hunter in 1757, the spectrum of underlying conditions associated with it has been shifted from tuberculous mediastinitis and syphilitic aortic aneurysms to malignant disorders. SVCS is rare in children and appears at presentation in 12% of pediatric patients with malignant mediastinal tumors.2 In adults, SVC syndrome is more common with small cell carcinoma. However, non small cell carcinoma is more frequently associated with SVCS because of its higher incidence. Both in adults and children, the most frequent non-malignant cause of SVCS is thrombosis from catheterization for central venous access.1 Superior vena cava syndrome (SVCS) refers to predominant major vessel compression in the superior mediastenum. When vessel compression is associated with tracheal compression, it is termed “superior mediastinal syndrome” (SMS).1 In pediatric practice SVCS and SMS are often used synonymously. In children, SVCS / SMS always constitutes a serious medical emergency, due to the presence of accompanying respiratory compromise. Although rare, mediastinal GCTs presenting with SVCS is reported in children.3 The common symptoms of SVCS include coughing, hoarseness, respiratory distress and chest pain. Other less common but more serious symptoms include fainting, anxiety, confusion, tiredness, headache, vision problems and sense of fullness in the ears.1
Treatment of SVCS depends on the etiology and the severity of the symptoms. The
histological diagnosis should be made prior to initiation of any radiation
therapy or chemotherapy because the specific therapy of malignant obstruction
mainly depends on tumor histology. Emergency symptomatic management may be
achieved by elevating the head and using corticosteroids and diuretics. Steroids
are used to treat respiratory compromise in SCVS for long time but there are no
definitive studies that prove its effectiveness. However, diuretics are
frequently used in the treatment of SVCS although they may ultimately cause
dehydration.4
GCTs are found in the gonads or extragonadal sites, mainly in or near the midline. The
mediastinum is the most frequent extragonadal location for GCTs,
other known extragonadal sites are
retroperitoneum, pineal gland, sacrococcygeal area, vagina, urinary bladder,
liver, nasopharynx, posterior cranial fossa and the face.5 Among medistenal tumors or cysts, GCTs are
found in 10-15% of cases.6 Sometimes, the medistenal GCT is found
with silent testicular primary tumor. In such cases, it is associated with retroperitoneal
lymphadenopathy. As in the studied patient, the mediastinum, antero-superior
mediastinum is the most common site for GCTs.
Medistenal GCT may bulges selectively into one hemithorax or another and can adhere to
the surrounding structures. Except mature cystic teratoma, which affects both
sexes equally, all mediastinum GCTs have shown a strong male predilection. Pure
extragonadal yolk sac tumors (YST) are extremely rare and mostly occur in the younger age
group.5 Similar to the study patient, the YST is commonly found
admixed with other germ cell elements. The study patient had hugely
elevated levels of serum
fetoprotein and beta-HCG; such types
of raised markers are seen in non-seminomatous malignant mixed GCTs.7
In early embryogenesis, fetal yolk sac is the source of physiological AFP.
Elevated AFP level in a patient with GCTs is associated with malignant yolk sac
component.
Surgical excision is the treatment of choice for non-seminomatous tumors. These tumors are radio-resistant while chemo-sensitive and various chemotherapy regimens are used, such as PVB (Cisplatin, Vinblastine, Bleomycin), PEB (Cisplatin, Etoposide, Bleomycin) and JEB (Carboplatin, Etoposide, Bleomycin).5 The response and disease control have improved dramatically with the advent of platinum based chemotherapy. On initial presentation, the study patient had extensive involvement and was unfit for surgery but showed significant improvement after completion of the first cycle of chemotherapy. Unfortunately, the patient was defaulted for long time and died after completion of delayed second cycle of chemotherapy.
CONCLUSIONSVCS is a rare but serious problem in pediatrics. Early etiological diagnosis and proper management may significantly improve the outcome.
We would like to thank MR for the concept and design, RB for supervising and diagnosing the patient, and also for managing and revising the manuscript. We also like to thank NP for radiological diagnosis and manuscript preparation, and SS for pathological diagnosis and literatures search. The authors reported no conflict of interest and no funding was received on this work.
-
Rheingold SR, Lange BJ. Oncologic emergencies. In: Pizzo PA, Poplack DG , Eds. Principles and practice of pediatric oncology, 4 th Ed Philadelphia. Lippincott Williams and Wilkins 2002: 1177-1203.
King RM, Telander RL, Smithson WA. Primary mediastinal tumors in children. Journal of Pediatric Surgery 1982; 17:512-520.
-
Roganovic J. Superior mediastinal syndrome in an infant caused by mediastinal teratoma: case report. Tumor 2006; 92:462-464.
-
Baker GL, Barnes HJ. Superior vena cava syndrome: etiology, diagnosis and treatment. American Journal of Critical Care 1992; 1:54-64.
-
Castleberry RP, Cushing B, Perlman E, Hawkins EP. Germ cell tumors. In: Pizzo PA, Poplack DG , Eds. Principles and practice of pediatric oncology, 4th Ed Philadelphia. Lippincott Williams and Wilkins 2002: 921-945.
-
Davis Rd Jr ,Odham HN Jr, Sabiston DC Jr. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis ,management and results. Ann thorac Surg 1987; 44:229-237.
-
Irie T, Watanabe H, Kawaoi A, Takeuchi J. Alpha-fetoprotein (AFP), human chorionic gonadotropin (HCG) and carcinoembriogenic antigen (CEA) demonstrated in the immature glands of mediastinal teratocarcinoma. A case report. Cancer 1982; 50:1160-1165.